Elucidating the Activation Mechanism of the Proton-sensing GPR68 Receptor
Abstract
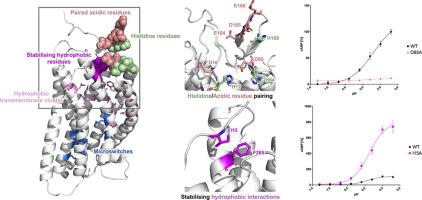
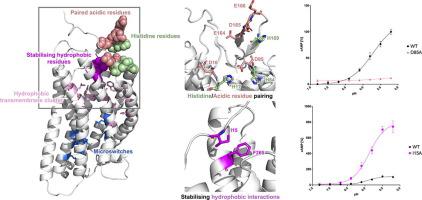
GPR68 is a proton-sensing G-protein Coupled Receptor (GPCR) involved in a variety of physiological processes and disorders including neoplastic pathologies. While GPR68 and few other GPCRs have been shown to be activated by a decrease in the extracellular pH, the molecular mechanism of their activation remains largely unknown. In this work, we used a combined computational and in vitro approach to provide new insight into the activation mechanism of the receptor. Molecular Dynamics simulations of GPR68 were used to model the changes in residue interactions and motions triggered by pH. Global and local rearrangements consistent with partial activation were observed upon protonation of the inactive state. Selected extracellular histidine and transmembrane acidic residues were found to have significantly upshifted pKa values during the simulations, consistently with their previously hypothesised role in activation through changes in protonation state. Moreover, a novel pairing between histidine and acidic residues in the extracellular region was highlighted by both sequence analyses and simulation data and tested through site-directed mutagenesis. At last, we identified a previously unknown hydrophobic lock in the extracellular region that might stabilise the inactive conformation and regulate the transition to the active state.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


