Jing Yang, James S Lewis, Jinghuan Zi, Thomas Andl, Ethan Lee, Claudia D Andl, Qi Liu, Robert D Beauchamp, Anna L Means
下载PDF
{"title":"Interaction of the tumor suppressor SMAD4 and WNT signaling in progression to oral squamous cell carcinoma","authors":"Jing Yang, James S Lewis, Jinghuan Zi, Thomas Andl, Ethan Lee, Claudia D Andl, Qi Liu, Robert D Beauchamp, Anna L Means","doi":"10.1002/path.6318","DOIUrl":null,"url":null,"abstract":"<p>SMAD4 is a tumor suppressor mutated or silenced in multiple cancers, including oral cavity squamous cell carcinoma (OSCC). Human clinical samples and cell lines, mouse models and organoid culture were used to investigate the role that SMAD4 plays in progression from benign disease to invasive OSCC. Human OSCC lost detectable SMAD4 protein within tumor epithelium in 24% of cases, and this loss correlated with worse progression-free survival independent of other major clinical and pathological features. A mouse model engineered for <i>Kras</i><sup>G12D</sup> expression in the adult oral epithelium induced benign papillomas, however the combination of <i>Kras</i><sup>G12D</sup> with loss of epithelial <i>Smad4</i> expression resulted in rapid development of invasive carcinoma with features of human OSCC. Examination of regulatory pathways in 3D organoid cultures of SMAD4+ and SMAD4− mouse tumors with <i>Kras</i> mutation found that either loss of SMAD4 or inhibition of TGFβ signaling upregulated the WNT pathway and altered the extracellular matrix. The gene signature of the mouse tumor organoids lacking SMAD4 was highly similar to the gene signature of human head and neck squamous cell carcinoma. In summary, this work has uncovered novel mechanisms by which SMAD4 acts as a tumor suppressor in OSCC. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 1","pages":"4-16"},"PeriodicalIF":5.6000,"publicationDate":"2024-06-26","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11300146/pdf/","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6318","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
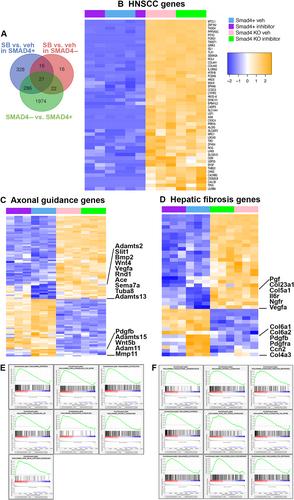
SMAD4 is a tumor suppressor mutated or silenced in multiple cancers, including oral cavity squamous cell carcinoma (OSCC). Human clinical samples and cell lines, mouse models and organoid culture were used to investigate the role that SMAD4 plays in progression from benign disease to invasive OSCC. Human OSCC lost detectable SMAD4 protein within tumor epithelium in 24% of cases, and this loss correlated with worse progression-free survival independent of other major clinical and pathological features. A mouse model engineered for Kras G12D expression in the adult oral epithelium induced benign papillomas, however the combination of Kras G12D with loss of epithelial Smad4 expression resulted in rapid development of invasive carcinoma with features of human OSCC. Examination of regulatory pathways in 3D organoid cultures of SMAD4+ and SMAD4− mouse tumors with Kras mutation found that either loss of SMAD4 or inhibition of TGFβ signaling upregulated the WNT pathway and altered the extracellular matrix. The gene signature of the mouse tumor organoids lacking SMAD4 was highly similar to the gene signature of human head and neck squamous cell carcinoma. In summary, this work has uncovered novel mechanisms by which SMAD4 acts as a tumor suppressor in OSCC. © 2024 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
肿瘤抑制因子 SMAD4 和 WNT 信号在口腔鳞状细胞癌发展过程中的相互作用。
SMAD4是一种在多种癌症(包括口腔鳞状细胞癌(OSCC))中发生突变或沉默的肿瘤抑制因子。研究人员利用人类临床样本和细胞系、小鼠模型和类器官培养物研究了SMAD4在良性疾病发展为浸润性OSCC过程中的作用。24%的人类OSCC病例的肿瘤上皮细胞中检测不到SMAD4蛋白,这种缺失与无进展生存期的恶化相关,与其他主要临床和病理特征无关。一种在成年口腔上皮细胞中表达 KrasG12D 的小鼠模型诱发了良性乳头状瘤,然而 KrasG12D 与上皮细胞 Smad4 表达缺失相结合,导致了具有人类 OSCC 特征的浸润性癌的快速发展。对Kras突变的SMAD4+和SMAD4-小鼠肿瘤的三维类器官培养物中的调控通路的研究发现,SMAD4的缺失或TGFβ信号的抑制都会上调WNT通路并改变细胞外基质。缺失SMAD4的小鼠肿瘤器官组织的基因特征与人类头颈部鳞状细胞癌的基因特征高度相似。总之,这项研究发现了SMAD4在OSCC中作为肿瘤抑制因子的新机制。© 2024 作者。病理学杂志》由 John Wiley & Sons Ltd 代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


