Facile synthesis of alkylphosphonates from 4-alkyl-1,4-dihydropyridines via photoinduced formal deformylative phosphonylation†
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Here, an unprecedented formal deformylative phosphonylation is disclosed. Under the catalysis of 1 mol% 1,2,3,5-tetrakis(diphenylamino)-4,6-dicyanobenzene (4DPAIPN) and in the presence of 200 mol% triethylamine as an additive, 4-alkyl-1,4-dihydropyridines (DHPs), derived from aldehydes, smoothly underwent phosphonylation with 9-fluorenyl o-phenylene phosphite, allowing facile synthesis of alkylphosphonates. This strategy is applicable to primary, secondary, and even tertiary DHPs and exhibits a broad substrate scope and excellent functional group tolerance, thereby enabling the late-stage phosphonylation of a series of naturally-occurring bioactive and biorelevant molecules.
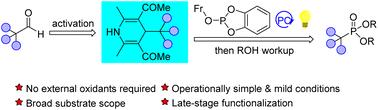
通过光诱导的形式变形磷酸化反应,从 4-烷基-1,4-二氢吡啶轻松合成烷基膦酸盐
这里公开了一种前所未有的形式变形膦酰化反应。在 1 mol % 1,2,3,5-四(二苯基氨基)-4,6-二氰基苯(4DPAIPN)和 200 mol % 三乙胺作为添加剂的催化下,由醛衍生的 4-烷基-1,4-二氢吡啶(DHPs)与 9-芴基邻苯亚磷酸酯顺利发生了膦酰化反应,从而轻松合成了烷基膦酸盐。这种策略适用于一级、二级甚至三级 DHP,具有广泛的底物范围和出色的官能团耐受性,因此可以对一系列天然生成的生物活性和生物相关分子进行后期膦酰化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


