Innate and adaptive immune responses that control lymph-borne viruses in the draining lymph node
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
The interstitial fluids in tissues are constantly drained into the lymph nodes (LNs) as lymph through afferent lymphatic vessels and from LNs into the blood through efferent lymphatics. LNs are strategically positioned and have the appropriate cellular composition to serve as sites of adaptive immune initiation against invading pathogens. However, for lymph-borne viruses, which disseminate from the entry site to other tissues through the lymphatic system, immune cells in the draining LN (dLN) also play critical roles in curbing systemic viral dissemination during primary and secondary infections. Lymph-borne viruses in tissues can be transported to dLNs as free virions in the lymph or within infected cells. Regardless of the entry mechanism, infected myeloid antigen-presenting cells, including various subtypes of dendritic cells, inflammatory monocytes, and macrophages, play a critical role in initiating the innate immune response within the dLN. This innate immune response involves cellular crosstalk between infected and bystander innate immune cells that ultimately produce type I interferons (IFN-Is) and other cytokines and recruit inflammatory monocytes and natural killer (NK) cells. IFN-I and NK cell cytotoxicity can restrict systemic viral spread during primary infections and prevent serious disease. Additionally, the memory CD8+ T-cells that reside or rapidly migrate to the dLN can contribute to disease prevention during secondary viral infections. This review explores the intricate innate immune responses orchestrated within dLNs that contain primary viral infections and the role of memory CD8+ T-cells following secondary infection or CD8+ T-cell vaccination.
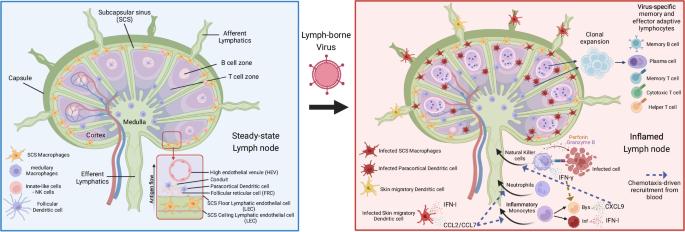
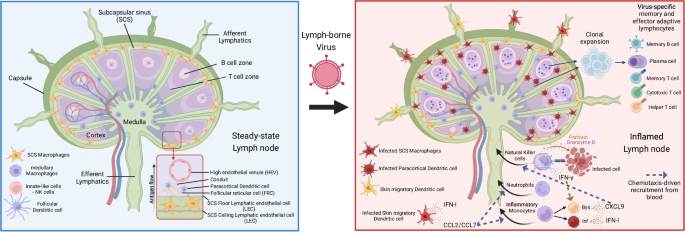
控制引流淋巴结中淋巴传播病毒的先天性和适应性免疫反应。
组织间隙液不断通过传入淋巴管以淋巴形式排入淋巴结(LN),并通过传出淋巴管从淋巴结进入血液。淋巴结位置优越,具有适当的细胞组成,可作为对抗入侵病原体的适应性免疫起始点。然而,对于通过淋巴系统从进入部位扩散到其他组织的淋巴传播病毒来说,引流淋巴结(dLN)中的免疫细胞在原发性和继发性感染期间也在遏制全身性病毒传播方面发挥着关键作用。组织中的淋巴传播病毒可作为淋巴中的游离病毒或感染细胞内的病毒被运送到引流淋巴结。无论是哪种进入机制,受感染的骨髓抗原递呈细胞,包括各种亚型的树突状细胞、炎性单核细胞和巨噬细胞,都在启动 dLN 内的先天性免疫反应中发挥着关键作用。这种先天性免疫反应涉及受感染的先天性免疫细胞和旁观者先天性免疫细胞之间的细胞串联,最终产生 I 型干扰素(IFN-Is)和其他细胞因子,并招募炎性单核细胞和自然杀伤细胞(NK)。IFN-I 和 NK 细胞的细胞毒性可在原发性感染期间限制病毒的全身扩散,防止严重疾病的发生。此外,驻留或快速迁移到 dLN 的记忆 CD8+ T 细胞也有助于在继发性病毒感染时预防疾病。这篇综述探讨了包含原发性病毒感染的 dLN 内错综复杂的先天性免疫反应,以及记忆 CD8+ T 细胞在继发性感染或 CD8+ T 细胞疫苗接种后的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


