P4HA2 hydroxylates SUFU to regulate the paracrine Hedgehog signaling and promote B-cell lymphoma progression
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
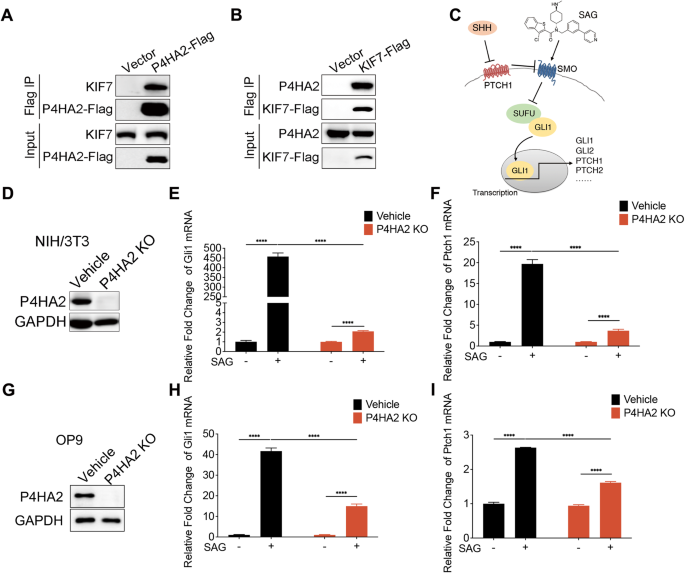
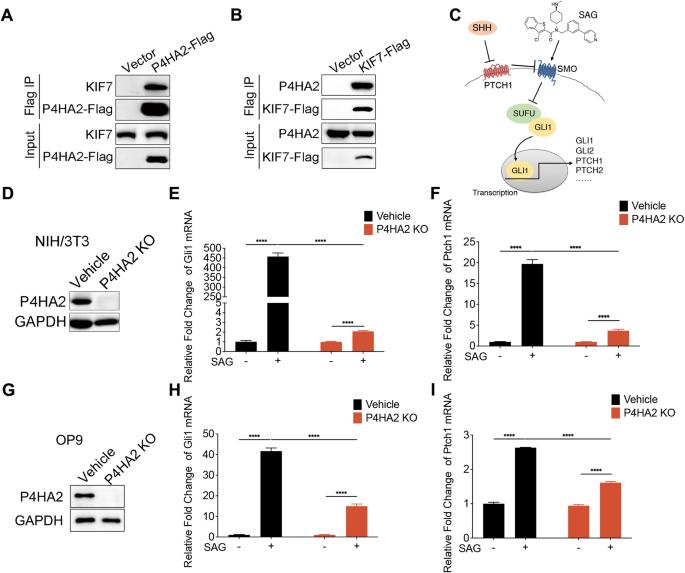
Aberrations in the Hedgehog (Hh) signaling pathway are significantly prevailed in various cancers, including B-cell lymphoma. A critical facet of Hh signal transduction involves the dynamic regulation of the suppressor of fused homolog (SUFU)-glioma-associated oncogene homolog (GLI) complex within the kinesin family member 7 (KIF7)-supported ciliary tip compartment. However, the specific post-translational modifications of SUFU-GLI complex within this context have remained largely unexplored. Our study reveals a novel regulatory mechanism involving prolyl 4-hydroxylase 2 (P4HA2), which forms a complex with KIF7 and is essential for signal transduction of Hh pathway. We demonstrate that, upon Hh pathway activation, P4HA2 relocates alongside KIF7 to the ciliary tip. Here, it hydroxylates SUFU to inhibit its function, thus amplifying the Hh signaling. Moreover, the absence of P4HA2 significantly impedes B lymphoma progression. This effect can be attributed to the suppression of Hh signaling in stromal fibroblasts, resulting in decreased growth factors essential for malignant proliferation of B lymphoma cells. Our findings highlight the role of P4HA2-mediated hydroxylation in modulating Hh signaling and propose a novel stromal-targeted therapeutic strategy for B-cell lymphoma.
P4HA2 羟基化 SUFU,调节旁分泌型刺猬信号,促进 B 细胞淋巴瘤的进展
在包括B细胞淋巴瘤在内的各种癌症中,刺猬(Hh)信号通路的畸变非常普遍。Hh信号转导的一个重要方面涉及融合同源物抑制因子(SUFU)-胶质瘤相关癌基因同源物(GLI)复合物在驱动蛋白家族成员7(KIF7)支持的纤毛末端区室中的动态调控。然而,SUFU-GLI 复合物在此背景下的特定翻译后修饰在很大程度上仍未被探索。我们的研究揭示了一种涉及脯氨酰 4-羟化酶 2(P4HA2)的新型调控机制,P4HA2 与 KIF7 形成复合物,对 Hh 通路的信号转导至关重要。我们证明,Hh 通路激活后,P4HA2 会与 KIF7 一起迁移到睫状末端。在这里,它羟化 SUFU 以抑制其功能,从而扩大了 Hh 信号转导。此外,P4HA2的缺失会显著阻碍B淋巴瘤的进展。这种效应可归因于基质成纤维细胞中的Hh信号被抑制,导致B淋巴瘤细胞恶性增殖所必需的生长因子减少。我们的研究结果突出了P4HA2-介导的羟化在调节Hh信号传导中的作用,并提出了一种新的B细胞淋巴瘤基质靶向治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


