SENP6 restricts the IFN-I-induced signaling pathway and antiviral activity by deSUMOylating USP8
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
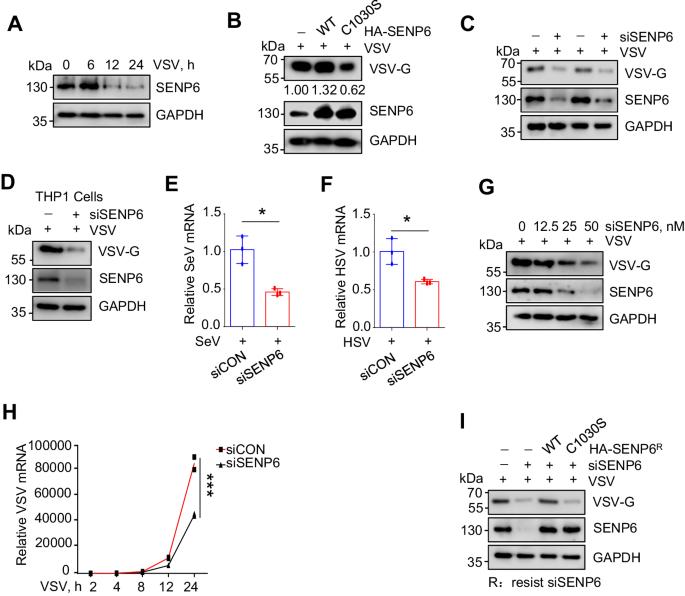
Type I interferon (IFN-I) exhibits broad-spectrum antiviral properties and is commonly employed in clinical for the treatment of viral infections. In this study, we unveil SENP6 as a potent regulator of IFN-I antiviral activity. SENP6 does not impact the production of IFN-I induced by viruses but rather modulates IFN-I-activated signaling. Mechanistically, SENP6 constitutively interacts with USP8 and inhibits the SUMOylation of USP8, consequently restricting the interaction between USP8 and IFNAR2. The dissociation of USP8 from IFNAR2 enhances IFNAR2 ubiquitination and degradation, thus attenuating IFN-I antiviral activity. Correspondingly, the downregulation of SENP6 promotes the interaction between USP8 and IFNAR2, leading to a reduction in IFNAR2 ubiquitination and, consequently, an enhancement in IFN-I-induced signaling. This study deciphers a critical deSUMOylation-deubiquitination crosstalk that finely regulates the IFN-I response to viral infection.
SENP6 通过去 SUMOylating USP8 限制 IFN-I 诱导的信号通路和抗病毒活性。
I 型干扰素(IFN-I)具有广谱抗病毒特性,临床上常用于治疗病毒感染。在这项研究中,我们发现 SENP6 是 IFN-I 抗病毒活性的有效调节因子。SENP6 不影响病毒诱导的 IFN-I 的产生,而是调节 IFN-I 激活的信号传导。从机制上讲,SENP6 与 USP8 构成性相互作用,抑制 USP8 的 SUMOylation,从而限制 USP8 与 IFNAR2 之间的相互作用。USP8 与 IFNAR2 的分离增强了 IFNAR2 的泛素化和降解,从而削弱了 IFN-I 的抗病毒活性。相应地,SENP6 的下调会促进 USP8 和 IFNAR2 之间的相互作用,导致 IFNAR2 泛素化减少,从而增强 IFN-I 诱导的信号传导。这项研究揭示了一个关键的去SUMOylation-泛素化串联过程,它精细地调节了IFN-I对病毒感染的反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


