Development of an effective predictive screening tool for prostate cancer using the ClarityDX machine learning platform
IF 12.4
1区 医学
Q1 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
Abstract
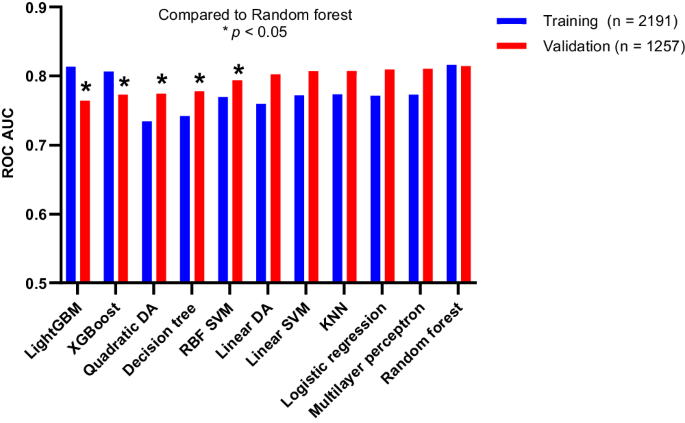
The current prostate cancer (PCa) screen test, prostate-specific antigen (PSA), has a high sensitivity for PCa but low specificity for high-risk, clinically significant PCa (csPCa), resulting in overdiagnosis and overtreatment of non-csPCa. Early identification of csPCa while avoiding unnecessary biopsies in men with non-csPCa is challenging. We built an optimized machine learning platform (ClarityDX) and showed its utility in generating models predicting csPCa. Integrating the ClarityDX platform with blood-based biomarkers for clinically significant PCa and clinical biomarker data from a 3448-patient cohort, we developed a test to stratify patients’ risk of csPCa; called ClarityDX Prostate. When predicting high risk cancer in the validation cohort, ClarityDX Prostate showed 95% sensitivity, 35% specificity, 54% positive predictive value, and 91% negative predictive value, at a ≥ 25% threshold. Using ClarityDX Prostate at this threshold could avoid up to 35% of unnecessary prostate biopsies. ClarityDX Prostate showed higher accuracy for predicting the risk of csPCa than PSA alone and the tested model-based risk calculators. Using this test as a reflex test in men with elevated PSA levels may help patients and their healthcare providers decide if a prostate biopsy is necessary.
利用 ClarityDX 机器学习平台开发有效的前列腺癌预测性筛查工具。
目前的前列腺癌(PCa)筛查测试--前列腺特异性抗原(PSA)--对 PCa 的灵敏度较高,但对高风险、有临床意义的 PCa(csPCa)的特异性较低,从而导致对非 csPCa 的过度诊断和过度治疗。在避免对患有非 csPCa 的男性进行不必要活检的同时,早期识别 csPCa 是一项挑战。我们建立了一个优化的机器学习平台(ClarityDX),并展示了其在生成预测 csPCa 模型方面的实用性。我们将 ClarityDX 平台与具有临床意义的 PCa 血液生物标记物以及来自 3448 例患者队列的临床生物标记物数据进行了整合,开发出了一种对患者的 csPCa 风险进行分层的测试方法,称为 ClarityDX Prostate。在预测验证队列中的高风险癌症时,ClarityDX Prostate 在≥ 25% 的阈值下显示出 95% 的灵敏度、35% 的特异性、54% 的阳性预测值和 91% 的阴性预测值。在此阈值下使用 ClarityDX Prostate 可以避免多达 35% 的不必要前列腺活检。在预测 csPCa 风险方面,ClarityDX Prostate 比单纯的 PSA 和经过测试的基于模型的风险计算器显示出更高的准确性。将该检测作为PSA水平升高的男性的反射性检测,可帮助患者及其医疗保健提供者决定是否有必要进行前列腺活检。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

NPJ Digital Medicine
Multiple-
CiteScore
25.10
自引率
3.30%
发文量
170
审稿时长
15 weeks
期刊介绍:
npj Digital Medicine is an online open-access journal that focuses on publishing peer-reviewed research in the field of digital medicine. The journal covers various aspects of digital medicine, including the application and implementation of digital and mobile technologies in clinical settings, virtual healthcare, and the use of artificial intelligence and informatics.
The primary goal of the journal is to support innovation and the advancement of healthcare through the integration of new digital and mobile technologies. When determining if a manuscript is suitable for publication, the journal considers four important criteria: novelty, clinical relevance, scientific rigor, and digital innovation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


