Retrospective study on pomalidomide-PACE as a salvage regimen in aggressive relapsed and refractory multiple myeloma
Abstract
Objectives
Despite major advances in treatment options for multiple myeloma (MM), patients refractory to the main drug classes and those with aggressive, especially extramedullary disease, still face a dismal outcome. For these patients, effective therapeutic options are urgently warranted.
Methods
In this retrospective study, we report on the safety and efficacy of the intensive combination regimen of pomalidomide plus cisplatin, doxorubicin, cyclophosphamide, and etoposide (Pom-PACE) in patients with relapsed refractory MM (RRMM) or plasma cell leukemia (PCL). A study population of 20 consecutive patients treated with Pom-PACE at two academic centers was included for analysis. All patients had to have a confirmed relapse according to International Myeloma Working Group criteria and adequate organ function prior to the start of therapy. Data were collected by reviewing medical charts. Exploratory analyses were performed with regard to efficacy and safety.
Results
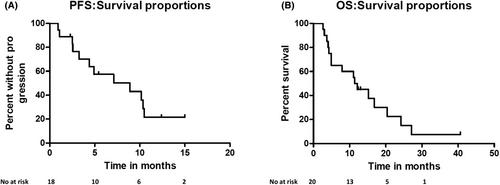
Patients were heavily pretreated with a median number of four prior therapies (range: 1–10). All patients were exposed to immunomodulators, proteasome inhibitors, and alkylating agents, 80% were double-class refractory, 40% were triple-class refractory. Extramedullary MM or PCL were present in 15 patients (75%). Overall response rate (ORR) was 68%, with 31% achieving at least a very good partial response. Responses were achieved rapidly with an ORR of 64% after one cycle. Median progression-free survival was 8.9 months (0.92–not reached [NR]) and median overall survival was 11.8 months (3–40.6). Pom-PACE was associated with significant toxicity. All evaluable patients experienced Grade 4 hematological toxicity. However, no treatment related mortality was observed.
Conclusion
Pomalidomide-PACE was able to induce rapid responses in heavily pretreated, aggressive RRMM with a manageable toxicity profile and therefore offers an effective salvage regimen and a potential bridging strategy to further treatment options such as chimeric antigen receptor T-cell therapy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


