Gut microbiota-mediated activation of GSDMD ignites colorectal tumorigenesis
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Activation of Gasdermin D (GSDMD) results in its cleavage, oligomerization, and subsequent formation of plasma membrane pores, leading to a form of inflammatory cell death denoted as pyroptosis. The roles of GSDMD in inflammation and immune responses to infection are well documented. However, whether GSDMD also plays a role in sporadic cancer development, especially that in the gut epithelium, remains unknown. Here, we show that GSDMD is activated in colorectal tumors of both human and mouse origins. Ablation of GSDMD in a mouse model of sporadic colorectal cancer resulted in reduced tumor formation in the colon and rectum, suggesting a tumor-promoting role of the protein in the gut. Both antibiotic-mediated depletion of gut microbiota and pharmacological inhibition of NLRP3 inflammasome reduced the activation of GSDMD. Loss of GSDMD resulted in reduced infiltration of immature myeloid cells, and increased numbers of macrophages in colorectal tumors. Activation of GSDMD is also accompanied by the aggregation of the endosomal sorting complex required for transport (ESCRT) membrane repair proteins on the membrane of colorectal tumor cells, suggesting that active membrane repairment may prevent pyroptosis induced by the formation of GSDMD pore in tumor cells. Our results show that gut microbiota/NLRP3-mediated activation of GSDMD promotes the development of colorectal tumors, and supports the use of NLRP3 inhibitors to treat colon cancer.
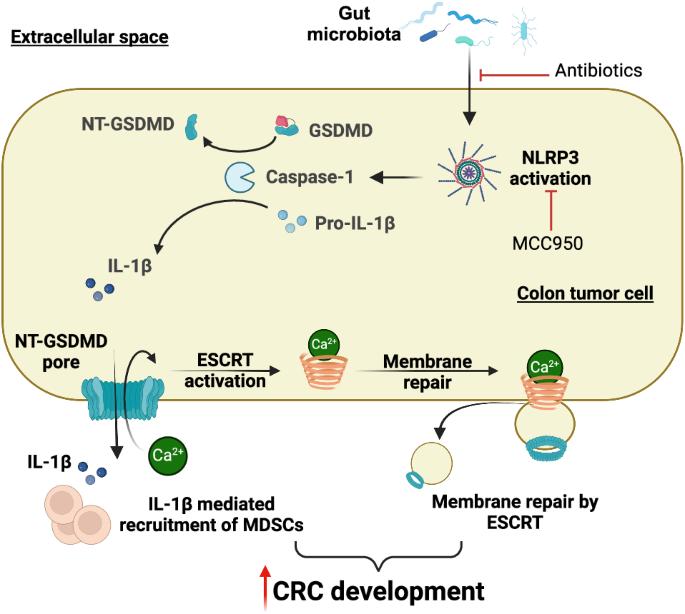
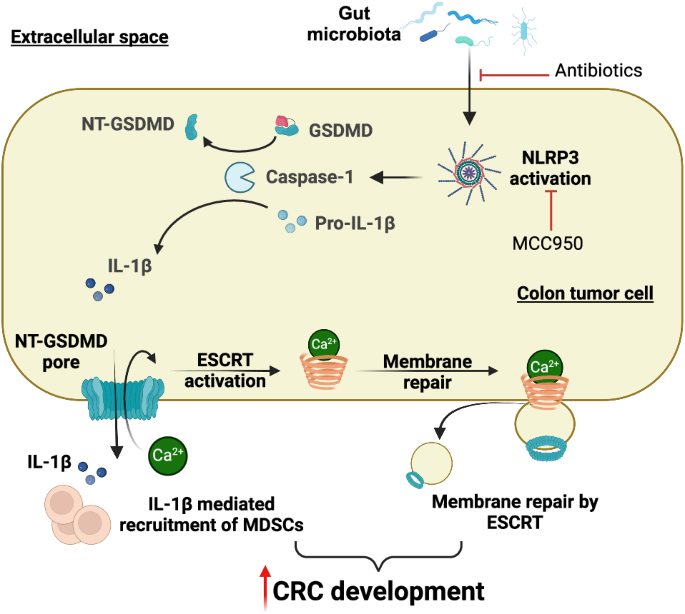
肠道微生物群介导的 GSDMD 激活引发结直肠肿瘤发生。
Gasdermin D(GSDMD)被激活后会发生裂解、寡聚化,随后形成质膜孔,导致一种炎症性细胞死亡,这种死亡形式被称为 "裂解热"(pyroptosis)。GSDMD 在炎症和感染免疫反应中的作用有据可查。然而,GSDMD 是否也在散发性癌症的发展中发挥作用,尤其是在肠道上皮细胞中,目前仍是未知数。在这里,我们发现 GSDMD 在人类和小鼠的结直肠肿瘤中都被激活。在散发性结直肠癌小鼠模型中消融 GSDMD 可减少结肠和直肠中肿瘤的形成,这表明该蛋白在肠道中具有促进肿瘤的作用。抗生素介导的肠道微生物群耗竭和药物抑制 NLRP3 炎症小体都降低了 GSDMD 的活化。GSDMD 的缺失导致结直肠肿瘤中未成熟髓系细胞浸润减少,巨噬细胞数量增加。GSDMD 的活化还伴随着结直肠肿瘤细胞膜上运输所需的内质体分选复合物(ESCRT)膜修复蛋白的聚集,这表明主动膜修复可能会阻止肿瘤细胞中 GSDMD 孔的形成所诱导的脓毒症。我们的研究结果表明,肠道微生物群/NLRP3 介导的 GSDMD 激活会促进结直肠肿瘤的发展,并支持使用 NLRP3 抑制剂治疗结肠癌。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


