Atg7 autophagy-independent role on governing neural stem cell fate could be potentially applied for regenerative medicine
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
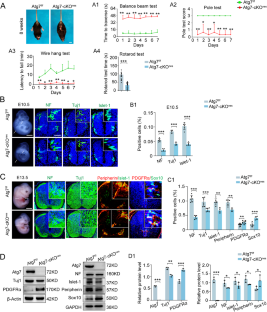
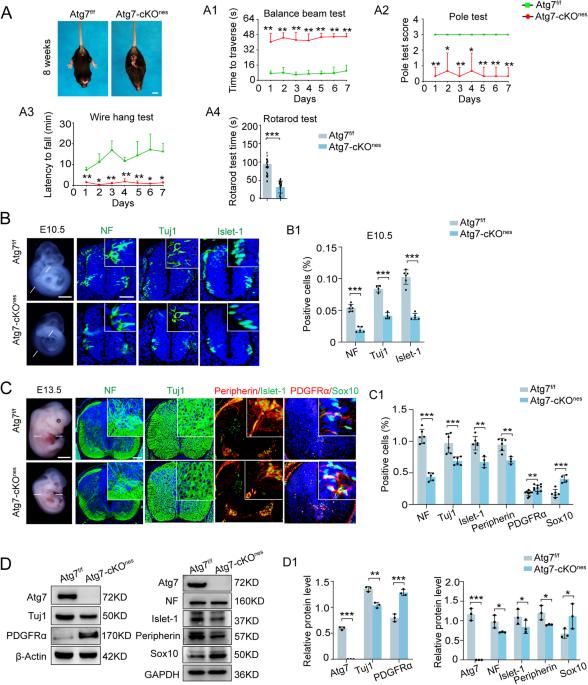
A literature review showed that Atg7 biological role was associated with the development and pathogenesis of nervous system, but very few reports focused on Atg7 role on neurogenesis at the region of spinal cord, so that we are committed to explore the subject. Atg7 expression in neural tube is incrementally increased during neurogenesis. Atg7 neural-specific knockout mice demonstrated the impaired motor function and imbalance of neuronal and glial cell differentiation during neurogenesis, which was similarly confirmed in primary neurosphere culture and reversely verified by Atg7 overexpression in unilateral neural tubes of gastrula chicken embryos. Furthermore, activating autophagy in neural stem cells (NSCs) of neurospheres did not rescue Atg7 deficiency-suppressed neuronal differentiation, but Atg7 overexpression on the basis of autophagy inhibition could reverse Atg7 deficiency-suppressed neuronal differentiation, which provides evidence for the existence of Atg7 role of autophagy-independent function. The underlying mechanism is that Atg7 deficiency directly caused the alteration of cell cycle length of NSCs, which is controlled by Atg7 through specifically binding Mdm2, thereby affecting neuronal differentiation during neurogenesis. Eventually, the effect of overexpressing Atg7-promoting neuronal differentiation was proved in spinal cord injury model as well. Taken together, this study revealed that Atg7 was involved in regulating neurogenesis by a non-autophagic signaling process, and this finding also shed light on the potential application in regenerative medicine.
独立于 Atg7 自噬的神经干细胞命运调控作用有望应用于再生医学
文献综述显示,Atg7的生物学作用与神经系统的发育和发病机制有关,但很少有报道关注Atg7在脊髓区域神经发生中的作用,因此我们致力于探讨这一主题。Atg7在神经管中的表达在神经发生过程中逐渐增加。Atg7神经特异性基因敲除小鼠在神经发生过程中表现出运动功能受损以及神经元和胶质细胞分化失衡,这一点在原代神经球培养中得到了类似的证实,而在单侧鸡胚胃神经管中Atg7的过表达则得到了反向验证。此外,激活神经球神经干细胞(NSCs)的自噬并不能挽救Atg7缺乏所抑制的神经元分化,但在抑制自噬的基础上过表达Atg7却能逆转Atg7缺乏所抑制的神经元分化,这为Atg7存在自噬依赖性功能提供了证据。其基本机制是Atg7缺乏直接导致了NSCs细胞周期长度的改变,而Atg7是通过特异性结合Mdm2来控制NSCs细胞周期长度的,从而影响了神经发生过程中神经元的分化。最终,在脊髓损伤模型中也证实了过表达Atg7促进神经元分化的作用。综上所述,这项研究揭示了Atg7通过非自噬信号转导过程参与调节神经发生,这一发现也为再生医学的潜在应用提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


