Nimodipine-associated standard dose reductions and neurologic outcomes after aneurysmal subarachnoid hemorrhage: the era of pharmacogenomics
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 0
Abstract
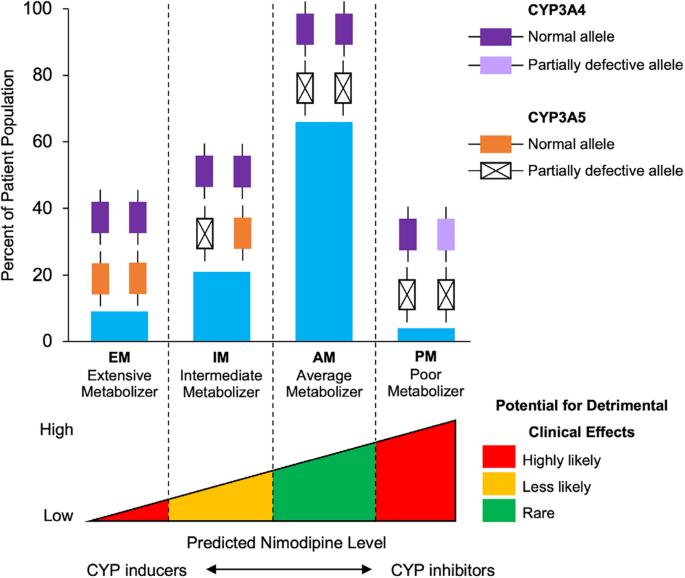
Nimodipine, an L-type cerebroselective calcium channel antagonist, is the only drug approved by the US Food and Drug Administration for the neuroprotection of patients with aneurysmal subarachnoid hemorrhage (aSAH). Four randomized, placebo-controlled trials of nimodipine demonstrated clinical improvement over placebo; however, these occurred before precision medicine with pharmacogenomics was readily available. The standard enteral dose of nimodipine recommended after aSAH is 60 mg every 4 h. However, up to 78% of patients with aSAH develop systemic arterial hypotension after taking the drug at the recommended dose, which could theoretically limit its neuroprotective role and worsen cerebral perfusion pressure and cerebral blood flow, particularly when concomitant vasospasm is present. We investigated the association between nimodipine dose changes and clinical outcomes in a consecutive series of 150 patients (mean age, 56 years; 70.7% women) with acute aSAH. We describe the pharmacogenomic relationship of nimodipine dose reduction with clinical outcomes. These results have major implications for future individualized dosing of nimodipine in the era of precision medicine.
动脉瘤性蛛网膜下腔出血后尼莫地平相关标准剂量的减少与神经系统预后:药物基因组学时代。
尼莫地平是一种 L 型脑选择性钙通道拮抗剂,是美国食品和药物管理局批准用于动脉瘤性蛛网膜下腔出血(aSAH)患者神经保护的唯一药物。尼莫地平的四项随机安慰剂对照试验显示,尼莫地平的临床疗效优于安慰剂;然而,这些试验都发生在药物基因组学精准医学刚刚问世之前。然而,高达 78% 的 aSAH 患者在按推荐剂量服药后会出现全身动脉低血压,这在理论上可能会限制尼莫地平的神经保护作用,并恶化脑灌注压和脑血流,尤其是在同时存在血管痉挛的情况下。我们连续研究了 150 例急性 aSAH 患者(平均年龄 56 岁,70.7% 为女性)的尼莫地平剂量变化与临床预后之间的关系。我们描述了尼莫地平剂量减少与临床结果之间的药物基因组学关系。这些结果对未来精准医疗时代尼莫地平的个体化剂量具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


