下载PDF
{"title":"The emerging role of targeted protein degradation to treat and study cancer","authors":"Maximillian H Brodermann, Elizabeth K Henderson, Rob S Sellar","doi":"10.1002/path.6301","DOIUrl":null,"url":null,"abstract":"<p>The evolution of cancer treatment has provided increasingly targeted strategies both in the upfront and relapsed disease settings. Small-molecule inhibitors and immunotherapy have risen to prominence with chimeric antigen receptor T-cells, checkpoint inhibitors, kinase inhibitors, and monoclonal antibody therapies being deployed across a range of solid organ and haematological malignancies. However, novel approaches are required to target transcription factors and oncogenic fusion proteins that are central to cancer biology and have generally eluded successful drug development. Thalidomide analogues causing protein degradation have been a cornerstone of treatment in multiple myeloma, but a lack of in-depth mechanistic understanding initially limited progress in the field. When the protein cereblon (CRBN) was found to mediate thalidomide analogues' action and CRBN's neo-targets were identified, existing and novel drug development accelerated, with applications outside multiple myeloma, including non-Hodgkin's lymphoma, myelodysplastic syndrome, and acute leukaemias. Critically, transcription factors were the first canonical targets described. In addition to broadening the application of protein-degrading drugs, resistance mechanisms are being overcome and targeted protein degradation is widening the scope of druggable proteins against which existing approaches have been ineffective. Examples of targeted protein degraders include molecular glues and proteolysis targeting chimeras (PROTACs): heterobifunctional molecules that bind to proteins of interest and cause proximity-induced ubiquitination and proteasomal degradation <i>via</i> a linked E3 ligase. Twenty years since their inception, PROTACs have begun progressing through clinical trials, with early success in targeting the oestrogen receptor and androgen receptor in breast and prostate cancer respectively. This review explores important developments in targeted protein degradation to both treat and study cancer. It also considers the potential advantages and challenges in the translational aspects of developing new treatments. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 4-5","pages":"403-417"},"PeriodicalIF":5.6000,"publicationDate":"2024-06-17","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6301","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6301","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
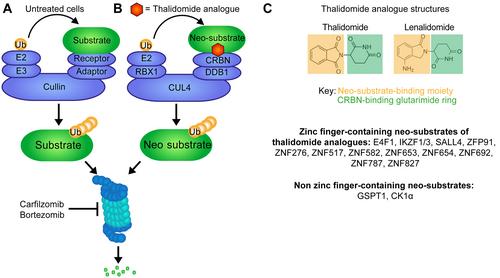
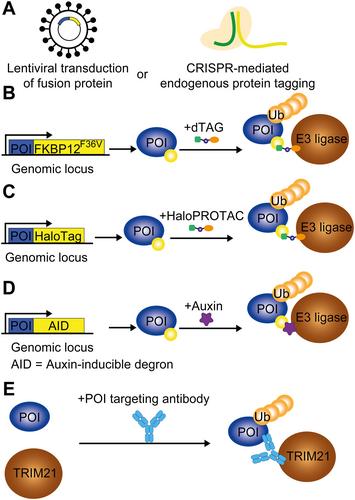
The evolution of cancer treatment has provided increasingly targeted strategies both in the upfront and relapsed disease settings. Small-molecule inhibitors and immunotherapy have risen to prominence with chimeric antigen receptor T-cells, checkpoint inhibitors, kinase inhibitors, and monoclonal antibody therapies being deployed across a range of solid organ and haematological malignancies. However, novel approaches are required to target transcription factors and oncogenic fusion proteins that are central to cancer biology and have generally eluded successful drug development. Thalidomide analogues causing protein degradation have been a cornerstone of treatment in multiple myeloma, but a lack of in-depth mechanistic understanding initially limited progress in the field. When the protein cereblon (CRBN) was found to mediate thalidomide analogues' action and CRBN's neo-targets were identified, existing and novel drug development accelerated, with applications outside multiple myeloma, including non-Hodgkin's lymphoma, myelodysplastic syndrome, and acute leukaemias. Critically, transcription factors were the first canonical targets described. In addition to broadening the application of protein-degrading drugs, resistance mechanisms are being overcome and targeted protein degradation is widening the scope of druggable proteins against which existing approaches have been ineffective. Examples of targeted protein degraders include molecular glues and proteolysis targeting chimeras (PROTACs): heterobifunctional molecules that bind to proteins of interest and cause proximity-induced ubiquitination and proteasomal degradation via a linked E3 ligase. Twenty years since their inception, PROTACs have begun progressing through clinical trials, with early success in targeting the oestrogen receptor and androgen receptor in breast and prostate cancer respectively. This review explores important developments in targeted protein degradation to both treat and study cancer. It also considers the potential advantages and challenges in the translational aspects of developing new treatments. © 2024 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
靶向蛋白质降解在治疗和研究癌症方面的新作用。
癌症治疗的发展为前期和复发疾病的治疗提供了越来越多的靶向策略。小分子抑制剂和免疫疗法已崭露头角,嵌合抗原受体 T 细胞、检查点抑制剂、激酶抑制剂和单克隆抗体疗法被广泛应用于各种实体器官和血液恶性肿瘤。然而,针对转录因子和致癌融合蛋白还需要新的方法,这些因子和蛋白是癌症生物学的核心,但一般都未能成功开发出药物。导致蛋白质降解的沙利度胺类似物一直是治疗多发性骨髓瘤的基石,但由于缺乏对机理的深入了解,最初限制了该领域的进展。当发现脑龙蛋白(CRBN)能介导沙利度胺类似物的作用,并确定了 CRBN 的新靶点后,现有药物和新药的开发速度加快,其应用领域已超出多发性骨髓瘤,包括非霍奇金淋巴瘤、骨髓增生异常综合征和急性白血病。至关重要的是,转录因子是第一个被描述的典型靶点。除了扩大蛋白降解药物的应用范围,抗药性机制也正在被克服,靶向蛋白降解正在扩大现有方法对其无效的可药用蛋白的范围。靶向蛋白降解剂的例子包括分子粘合剂和蛋白水解靶向嵌合体(PROTACs):与相关蛋白结合的杂多功能分子,通过连接的 E3 连接酶引起近似诱导泛素化和蛋白酶体降解。PROTACs问世二十年来,已开始在临床试验中取得进展,并分别在靶向乳腺癌雌激素受体和前列腺癌雄激素受体方面取得了早期成功。本综述探讨了靶向蛋白质降解在治疗和研究癌症方面的重要发展。它还探讨了开发新治疗方法在转化方面的潜在优势和挑战。作者:© 2024病理学杂志》由 John Wiley & Sons Ltd 代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


