Intravenous delivery of STING agonists using acid-sensitive polycationic polymer-modified lipid nanoparticles for enhanced tumor immunotherapy
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Although cancer immunotherapy has made great strides in the clinic, it is still hindered by the tumor immunosuppressive microenvironment (TIME). The stimulator of interferon genes (STING) pathway which can modulate TIME effectively has emerged as a promising therapeutic recently. However, the delivery of most STING agonists, specifically cyclic dinucleotides (CDNs), is performed intratumorally due to their insufficient pharmacological properties, such as weak permeability across cell membranes and vulnerability to nuclease degradation. To expand the clinical applicability of CDNs, a novel pH-sensitive polycationic polymer-modified lipid nanoparticle (LNP-B) system was developed for intravenous delivery of CDNs. LNP-B significantly extended the circulation of CDNs and enhanced the accumulation of CDNs within the tumor, spleen, and tumor-draining lymph nodes compared with free CDNs thereby triggering the STING pathway of dendritic cells and repolarizing pro-tumor macrophages. These events subsequently gave rise to potent anti-tumor immune reactions and substantial inhibition of tumors in CT26 colon cancer-bearing mouse models. In addition, due to the acid-sensitive property of the polycationic polymer, the delivery system of LNP-B was more biocompatible and safer compared with lipid nanoparticles formulated with an indissociable cationic DOTAP (LNP-D). These findings suggest that LNP-B has great potential in the intravenous delivery of CDNs for tumor immunotherapy.
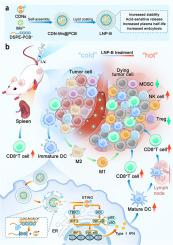

利用酸敏感聚阳离子聚合物修饰的脂质纳米粒子静脉注射 STING 激动剂,增强肿瘤免疫疗法的效果
尽管肿瘤免疫治疗在临床上取得了长足的进步,但仍受到肿瘤免疫抑制微环境(TIME)的阻碍。干扰素基因刺激因子(STING)通路能够有效调节时间,近年来已成为一种很有前景的治疗方法。然而,大多数STING激动剂,特别是环二核苷酸(cdn),由于其不充分的药理学性质,如细胞膜渗透性弱和易受核酸酶降解的影响,只能在瘤内给药。为了扩大cdn的临床适用性,研究人员开发了一种新型的ph敏感聚阳离子聚合物修饰脂质纳米颗粒(LNP-B)系统,用于静脉给药cdn。与游离cdn相比,LNP-B显著延长了cdn的循环,增强了cdn在肿瘤、脾脏和肿瘤引流淋巴结内的积累,从而触发树突状细胞的STING途径和肿瘤前巨噬细胞的再极化。这些事件随后在CT26结肠癌小鼠模型中引起了有效的抗肿瘤免疫反应和实质性的肿瘤抑制。此外,由于聚阳离子聚合物的酸敏感性,LNP-B的递送系统比由不可分离的阳离子DOTAP (LNP-D)组成的脂质纳米颗粒更具生物相容性和安全性。这些发现表明LNP-B在静脉给药cdn用于肿瘤免疫治疗方面具有很大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
文献相关原料
公司名称
产品信息
索莱宝
Amiloride
上海源叶
Dialysis bag
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


