Latest Developments in Palladium and Nickel-Catalyzed Cross-Couplings for Aryl Chlorides: Suzuki-Miyaura and Buchwald-Hartwig Reactions
引用次数: 0
Abstract
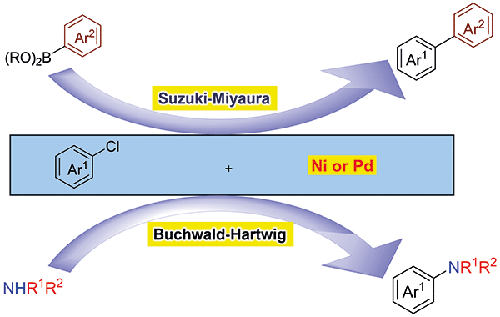
Palladium- and nickel-catalyzed cross-couplings are powerful methods for constructing C-C and C-N bonds, particularly through Suzuki-Miyaura and Buchwald-Hartwig reactions. Although aryl iodides, bromides, and triflates are the most commonly used substrates, aryl chlorides are less frequently utilized due to their lower reactivity. However, they are appealing because they are readily available and inexpensive. This short review highlights recent developments in Suzuki-Miyaura and Buchwald-Hartwig cross-couplings of aryl chlorides, using both homogeneous and heterogeneous catalysis with palladium and nickel.
钯和镍催化的芳基氯化物交叉耦合反应的最新进展:Suzuki-Miyaura和Buchwald-Hartwig反应
钯和镍催化的交叉偶联反应是构建 C-C 和 C-N 键的有效方法,特别是通过铃木-宫浦反应和布赫瓦尔德-哈特维格反应。虽然芳基碘化物、溴化物和三氟化物是最常用的底物,但芳基氯化物由于反应活性较低而较少使用。不过,由于芳基氯化物易于获得且价格低廉,因此很有吸引力。这篇简短的综述重点介绍了利用钯和镍的均相催化和异相催化进行芳基氯化物的铃木-宫脲和布赫瓦尔德-哈特维格交叉耦合反应的最新进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


