Regulation of primary cilia disassembly through HUWE1-mediated TTBK2 degradation plays a crucial role in cerebellar development and medulloblastoma growth
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
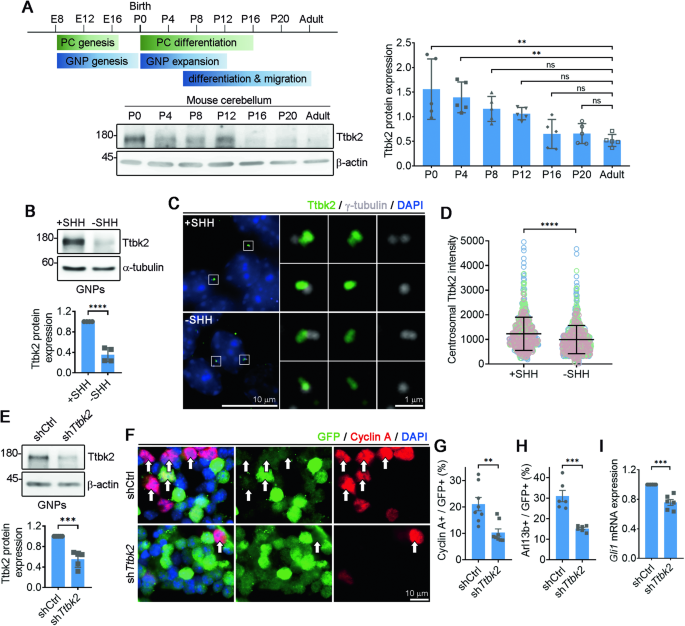
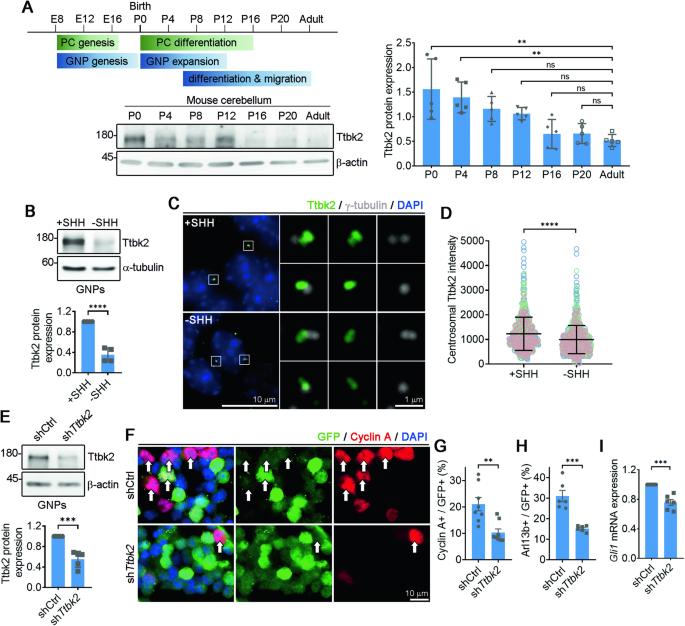
Development of the cerebellum requires precise regulation of granule neuron progenitor (GNP) proliferation. Although it is known that primary cilia are necessary to support GNP proliferation, the exact molecular mechanism governing primary cilia dynamics within GNPs remains elusive. Here, we establish the pivotal roles for the centrosomal kinase TTBK2 (Tau tubulin kinase-2) and the E3 ubiquitin ligase HUWE1 in GNP proliferation. We show that TTBK2 is highly expressed in proliferating GNPs under Sonic Hedgehog (SHH) signaling, coinciding with active GNP proliferation and the presence of primary cilia. TTBK2 stabilizes primary cilia by inhibiting their disassembly, thereby promoting GNP proliferation in response to SHH. Mechanistically, we identify HUWE1 as a novel centrosomal E3 ligase that facilitates primary cilia disassembly by targeting TTBK2 degradation. Disassembly of primary cilia serves as a trigger for GNP differentiation, allowing their migration from the external granule layer (EGL) of the cerebellum to the internal granule layer (IGL) for subsequent maturation. Moreover, we have established a link between TTBK2 and SHH-type medulloblastoma (SHH-MB), a tumor characterized by uncontrolled GNP proliferation. TTBK2 depletion inhibits SHH-MB proliferation, indicating that TTBK2 may be a potential therapeutic target for this cancer type. In summary, our findings reveal the mechanism governing cerebellar development and highlight a potential anti-cancer strategy for SHH-MB.
通过HUWE1介导的TTBK2降解来调节初级纤毛的分解在小脑发育和髓母细胞瘤生长中起着至关重要的作用。
小脑的发育需要对颗粒神经元祖细胞(GNP)的增殖进行精确调控。尽管人们知道初级纤毛是支持 GNP 增殖的必要条件,但支配 GNP 内初级纤毛动态的确切分子机制仍然难以确定。在这里,我们确定了中心体激酶 TTBK2(Tau tubulin kinase-2)和 E3 泛素连接酶 HUWE1 在 GNP 增殖中的关键作用。我们的研究表明,在音速刺猬(SHH)信号的作用下,TTBK2 在增殖的 GNP 中高度表达,这与 GNP 的增殖活跃和初级纤毛的存在相吻合。TTBK2 通过抑制初级纤毛的解体来稳定初级纤毛,从而促进 GNP 对 SHH 的增殖。从机理上讲,我们发现 HUWE1 是一种新型的中心粒 E3 连接酶,它通过靶向 TTBK2 降解来促进原生纤毛的解体。初级纤毛的解体是GNP分化的触发器,它允许GNP从小脑的外部颗粒层(EGL)迁移到内部颗粒层(IGL),以便随后成熟。此外,我们还建立了 TTBK2 与 SHH 型髓母细胞瘤(SHH-MB)之间的联系,SHH-MB 是一种以 GNP 不受控制地增殖为特征的肿瘤。TTBK2 的耗竭抑制了 SHH-MB 的增殖,这表明 TTBK2 可能是该癌症类型的潜在治疗靶点。总之,我们的研究结果揭示了小脑发育的机制,并强调了针对 SHH-MB 的潜在抗癌策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


