Lactylation of NAT10 promotes N4‐acetylcytidine modification on tRNASer-CGA-1-1 to boost oncogenic DNA virus KSHV reactivation
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
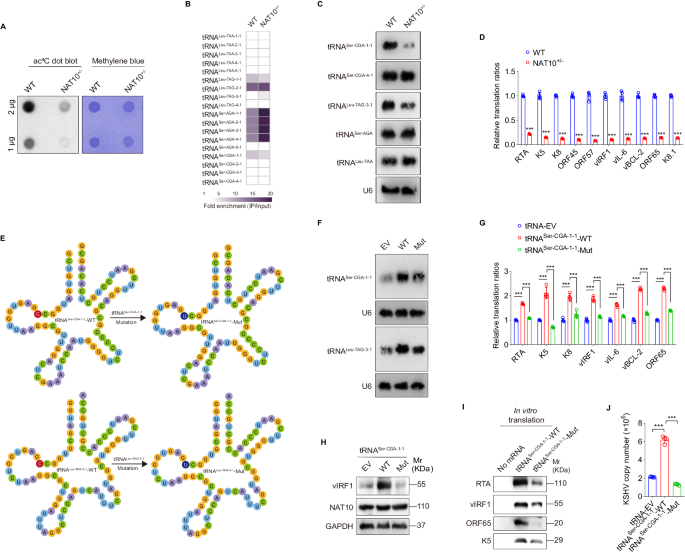
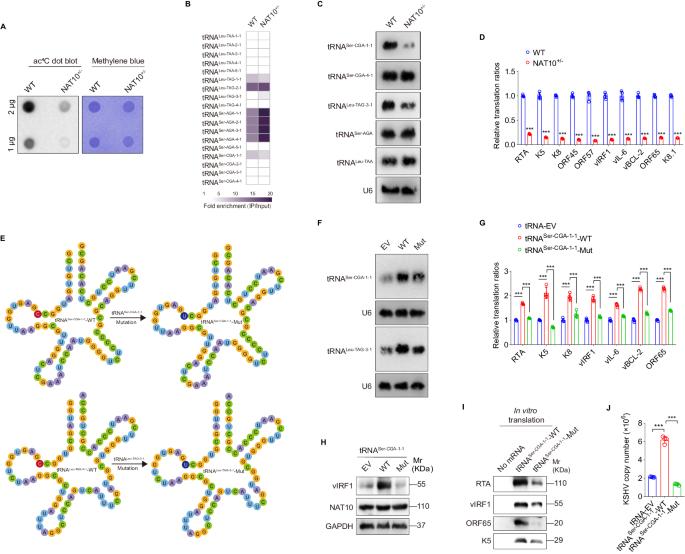
N4-acetylcytidine (ac4C), a conserved but recently rediscovered RNA modification on tRNAs, rRNAs and mRNAs, is catalyzed by N-acetyltransferase 10 (NAT10). Lysine acylation is a ubiquitous protein modification that controls protein functions. Our latest study demonstrates a NAT10-dependent ac4C modification, which occurs on the polyadenylated nuclear RNA (PAN) encoded by oncogenic DNA virus Kaposi’s sarcoma-associated herpesvirus (KSHV), can induce KSHV reactivation from latency and activate inflammasome. However, it remains unclear whether a novel lysine acylation occurs in NAT10 during KSHV reactivation and how this acylation of NAT10 regulates tRNAs ac4C modification. Here, we showed that NAT10 was lactylated by α-tubulin acetyltransferase 1 (ATAT1), as a writer at the critical domain, to exert RNA acetyltransferase function and thus increase the ac4C level of tRNASer-CGA-1-1. Mutagenesis at the ac4C site in tRNASer-CGA-1-1 inhibited its ac4C modifications, translation efficiency of viral lytic genes, and virion production. Mechanistically, KSHV PAN orchestrated NAT10 and ATAT1 to enhance NAT10 lactylation, resulting in tRNASer-CGA-1-1 ac4C modification, eventually boosting KSHV reactivation. Our findings reveal a novel post-translational modification in NAT10, as well as expand the understanding about tRNA-related ac4C modification during KSHV replication, which may be exploited to design therapeutic strategies for KSHV-related diseases.
NAT10的乳化作用促进tRNASer-CGA-1-1上的N4-乙酰胞苷修饰,从而促进致癌DNA病毒KSHV的再活化
N4-acetylcytidine (ac4C)是 tRNA、rRNA 和 mRNA 上的一种保守但最近被重新发现的 RNA 修饰,由 N-acetyltransferase 10 (NAT10) 催化。赖氨酸酰化是一种无处不在的控制蛋白质功能的蛋白质修饰。我们的最新研究表明,致癌 DNA 病毒卡波西肉瘤相关疱疹病毒(KSHV)编码的多聚腺苷酸核 RNA(PAN)上发生的依赖 NAT10 的 ac4C 修饰可诱导 KSHV 从潜伏期重新活化并激活炎性体。然而,目前仍不清楚在 KSHV 再激活过程中 NAT10 是否会发生新的赖氨酸酰化,以及 NAT10 的酰化如何调节 tRNA 的 ac4C 修饰。在这里,我们发现 NAT10 被 α-管蛋白乙酰转移酶 1(ATAT1)作为临界域的作者进行了乳酰化,以发挥 RNA 乙酰转移酶的功能,从而提高 tRNASer-CGA-1-1 的 ac4C 水平。tRNASer-CGA-1-1中ac4C位点的突变抑制了其ac4C修饰、病毒裂殖基因的翻译效率以及病毒的产生。从机理上讲,KSHV PAN协调NAT10和ATAT1增强NAT10的乳化作用,导致tRNASer-CGA-1-1的ac4C修饰,最终促进了KSHV的再活化。我们的研究结果揭示了 NAT10 的一种新的翻译后修饰,同时也拓展了人们对 KSHV 复制过程中与 tRNA 相关的 ac4C 修饰的认识,这可能会被用于设计 KSHV 相关疾病的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


