A. S. Jijumon, Arya Krishnan, Carsten Janke
{"title":"A Platform for Medium-Throughput Cell-Free Analyses of Microtubule-Interacting Proteins Using Mammalian Cell Lysates","authors":"A. S. Jijumon, Arya Krishnan, Carsten Janke","doi":"10.1002/cpz1.1070","DOIUrl":null,"url":null,"abstract":"<p>The microtubule (MT) cytoskeleton performs a variety of functions in cell division, cell architecture, neuronal differentiation, and ciliary beating. These functions are controlled by proteins that directly interact with MTs, commonly referred to as microtubule-associated proteins (MAPs). Out of the many proteins reported interact with MTs, only a some have been biochemically and functionally characterized so far. One of the limitations of classical in vitro assays and single-MT reconstitution approaches is that they are typically performed with purified proteins. As purification of proteins can be difficult and time-consuming, many previous studies have only focused on a few proteins, while systematic analyses of many different proteins by in vitro reconstitution assays were not possible. Here we present a detailed protocol using lysates of mammalian cells instead of purified proteins that overcomes this limitation. Those lysates contain all molecular components required for in vitro MT reconstitution including the endogenous tubulin and the recombinant MAPs, which form MT assemblies upon the injection of the lysates into a microscopy chamber. This allows to directly observe the dynamic behavior of growing MTs, as well as the fluorescently labeled associated proteins by total internal reflection fluorescence (TIRF) microscopy. Strikingly, all proteins tested so far were functional in our approach, thus providing the possibility to test virtually any protein of interest. This also opens the possibility to screen the impact of patient mutations on the MT binding behavior of MAPs in a medium-throughput manner. In addition, the lysate approach can easily be adapted to other applications that have predominantly been performed with purified proteins so far, such as investigating other cytoskeletal systems and cytoskeletal crosstalk, or to study structures of MAPs bound to MTs by cryo-electron microscopy. Our approach is thus a versatile, expandable, and easy-to-use method to characterize the impact of a broad spectrum of proteins on cytoskeletal behavior and function. © 2024 The Authors. Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Preparation of lysates of human cells for TIRF reconstitution assays</p><p><b>Basic Protocol 2</b>: Quantification of GFP-tagged MAP concentration in cell lysates</p><p><b>Support Protocol 1</b>: Purification of KIF5B(N555/T92A) (dead kinesin) protein for TIRF reconstitution assays</p><p><b>Support Protocol 2</b>: Preparation of GMPCPP MT seeds for TIRF reconstitution assays</p><p><b>Basic Protocol 3</b>: TIRF-based MT-MAP reconstitution assays using cell lysates</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 6","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2024-06-12","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1070","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.1070","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Abstract
The microtubule (MT) cytoskeleton performs a variety of functions in cell division, cell architecture, neuronal differentiation, and ciliary beating. These functions are controlled by proteins that directly interact with MTs, commonly referred to as microtubule-associated proteins (MAPs). Out of the many proteins reported interact with MTs, only a some have been biochemically and functionally characterized so far. One of the limitations of classical in vitro assays and single-MT reconstitution approaches is that they are typically performed with purified proteins. As purification of proteins can be difficult and time-consuming, many previous studies have only focused on a few proteins, while systematic analyses of many different proteins by in vitro reconstitution assays were not possible. Here we present a detailed protocol using lysates of mammalian cells instead of purified proteins that overcomes this limitation. Those lysates contain all molecular components required for in vitro MT reconstitution including the endogenous tubulin and the recombinant MAPs, which form MT assemblies upon the injection of the lysates into a microscopy chamber. This allows to directly observe the dynamic behavior of growing MTs, as well as the fluorescently labeled associated proteins by total internal reflection fluorescence (TIRF) microscopy. Strikingly, all proteins tested so far were functional in our approach, thus providing the possibility to test virtually any protein of interest. This also opens the possibility to screen the impact of patient mutations on the MT binding behavior of MAPs in a medium-throughput manner. In addition, the lysate approach can easily be adapted to other applications that have predominantly been performed with purified proteins so far, such as investigating other cytoskeletal systems and cytoskeletal crosstalk, or to study structures of MAPs bound to MTs by cryo-electron microscopy. Our approach is thus a versatile, expandable, and easy-to-use method to characterize the impact of a broad spectrum of proteins on cytoskeletal behavior and function. © 2024 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1: Preparation of lysates of human cells for TIRF reconstitution assays
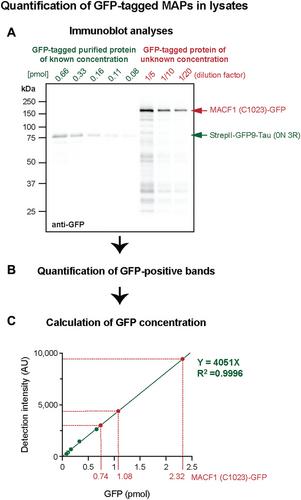
Basic Protocol 2: Quantification of GFP-tagged MAP concentration in cell lysates
Support Protocol 1: Purification of KIF5B(N555/T92A) (dead kinesin) protein for TIRF reconstitution assays
Support Protocol 2: Preparation of GMPCPP MT seeds for TIRF reconstitution assays
Basic Protocol 3: TIRF-based MT-MAP reconstitution assays using cell lysates
利用哺乳动物细胞裂解液进行微管相互作用蛋白中通量无细胞分析的平台
微管(MT)细胞骨架在细胞分裂、细胞结构、神经元分化和纤毛跳动中发挥着多种功能。这些功能由与 MT 直接相互作用的蛋白质控制,这些蛋白质通常被称为微管相关蛋白(MAPs)。据报道,在与 MTs 相互作用的众多蛋白质中,迄今只有部分蛋白质具有生物化学和功能特征。经典体外检测和单个 MT 重组方法的局限性之一是,它们通常是用纯化的蛋白质进行的。由于纯化蛋白质既困难又耗时,以前的许多研究只关注少数几种蛋白质,而无法通过体外重组实验对许多不同的蛋白质进行系统分析。在这里,我们介绍一种使用哺乳动物细胞裂解液而不是纯化蛋白质的详细方案,它克服了这一限制。这些裂解物含有体外 MT 重组所需的所有分子成分,包括内源性微管蛋白和重组 MAPs,将裂解物注入显微镜室后即可形成 MT 组装。这样就可以通过全内反射荧光(TIRF)显微镜直接观察生长中的 MT 的动态行为以及荧光标记的相关蛋白。令人吃惊的是,迄今为止测试的所有蛋白质在我们的方法中都是功能性的,因此几乎可以测试任何感兴趣的蛋白质。这也为以中等通量的方式筛选患者突变对 MAP 的 MT 结合行为的影响提供了可能。此外,裂解物方法还能轻松应用于迄今为止主要使用纯化蛋白进行的其他应用,如研究其他细胞骨架系统和细胞骨架串扰,或通过冷冻电镜研究与 MT 结合的 MAPs 结构。因此,我们的方法是一种多功能、可扩展且易于使用的方法,可用于表征各种蛋白质对细胞骨架行为和功能的影响。© 2024 作者。当前协议》由 Wiley Periodicals LLC 出版。基本方案 1:制备用于 TIRF 重组实验的人体细胞裂解液 基本方案 2:细胞裂解液中 GFP 标记的 MAP 浓度定量 支持方案 1:纯化用于 TIRF 重组实验的 KIF5B(N555/T92A)(死驱动蛋白)蛋白 支持方案 2:制备用于 TIRF 重组实验的 GMPCPP MT 种子 基本方案 3:使用细胞裂解液进行基于 TIRF 的 MT-MAP 重组实验。
本文章由计算机程序翻译,如有差异,请以英文原文为准。


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


