Anaplastic Lymphoma Kinase signaling stabilizes SLC3A2 expression via MARCH11 to promote neuroblastoma cell growth
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
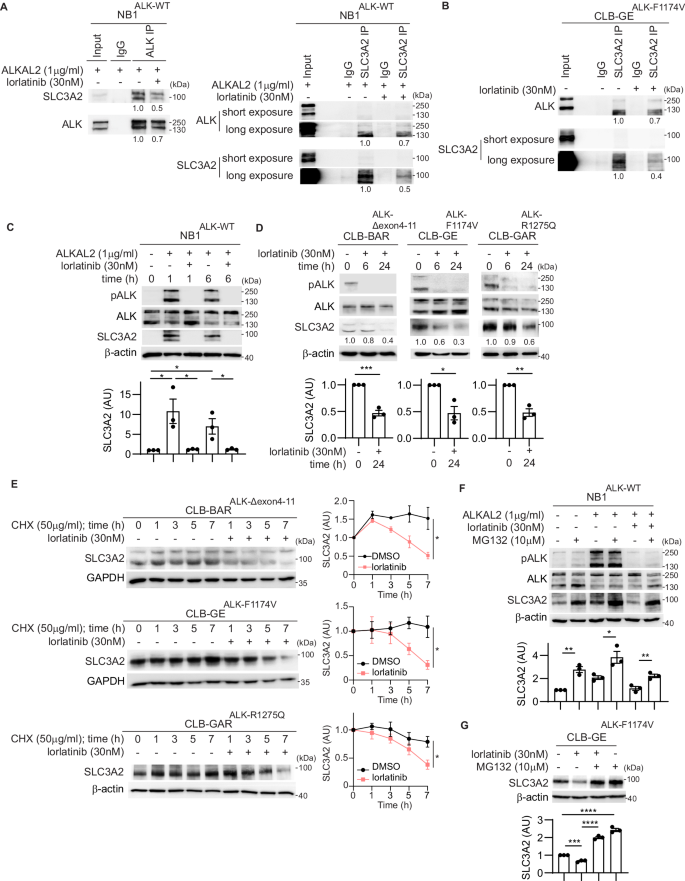
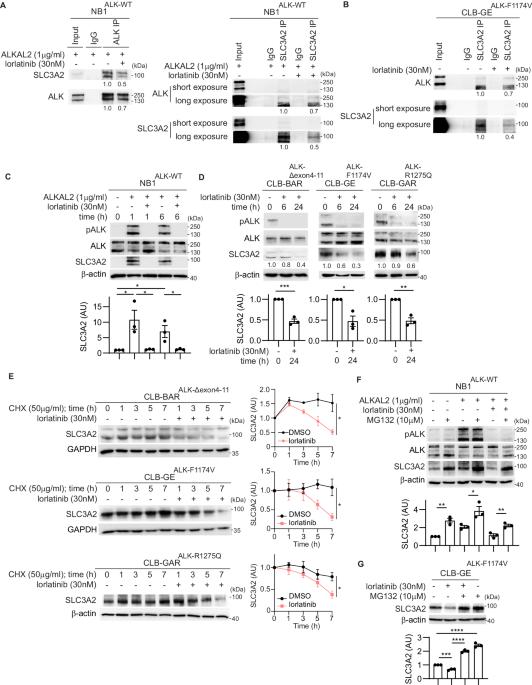
Solute Carrier Family 3, Member 2 (SLC3A2 or 4F2hc) is a multifunctional glycoprotein that mediates integrin-dependent signaling, acts as a trafficking chaperone for amino acid transporters, and is involved in polyamine transportation. We identified SLC3A2 as a potential Anaplastic Lymphoma Kinase (ALK) interacting partner in a BioID-proximity labeling screen in neuroblastoma (NB) cells. In this work we show that endogenous SLC3A2 and ALK interact in NB cells and that this SLC3A2:ALK interaction was abrogated upon treatment with the ALK inhibitor lorlatinib. We show here that loss of ALK activity leads to decreased SLC3A2 expression and reduced SLC3A2 protein stability in a panel of NB cell lines, while stimulation of ALK with ALKAL2 ligand resulted in increased SLC3A2 protein levels. We further identified MARCH11, an E3 ligase, as a regulator of SLC3A2 ubiquitination downstream of ALK. Further, knockdown of SLC3A2 resulted in inhibition of NB cell growth. To investigate the therapeutic potential of SLC3A2 targeting, we performed monotreatment of NB cells with AMXT-1501 (a polyamine transport inhibitor), which showed only moderate effects in NB cells. In contrast, a combination lorlatinib/AMXT-1501 treatment resulted in synergistic inhibition of cell growth in ALK-driven NB cell lines. Taken together, our results identify a novel role for the ALK receptor tyrosine kinase (RTK), working in concert with the MARCH11 E3 ligase, in regulating SLC3A2 protein stability and function in NB cells. The synergistic effect of combined ALK and polyamine transport inhibition shows that ALK/MARCH11/SLC3A2 regulation of amino acid transport is important for oncogenic growth and survival in NB cells.
无性淋巴瘤激酶信号通过 MARCH11 稳定 SLC3A2 的表达,从而促进神经母细胞瘤细胞的生长。
溶质运载家族 3 成员 2(SLC3A2 或 4F2hc)是一种多功能糖蛋白,可介导整合素依赖性信号传导,充当氨基酸转运体的转运伴侣,并参与多胺转运。我们在神经母细胞瘤(NB)细胞的生物识别-接近标记筛选中发现 SLC3A2 是一种潜在的无性淋巴瘤激酶(ALK)相互作用伙伴。在这项工作中,我们发现内源性 SLC3A2 和 ALK 在 NB 细胞中相互作用,而且这种 SLC3A2:ALK 相互作用在使用 ALK 抑制剂洛拉替尼处理后会减弱。我们在此表明,ALK活性丧失会导致SLC3A2表达减少,并降低NB细胞系中SLC3A2蛋白的稳定性,而用ALKAL2配体刺激ALK会导致SLC3A2蛋白水平升高。我们进一步发现,E3 连接酶 MARCH11 是 ALK 下游 SLC3A2 泛素化的调节因子。此外,敲除 SLC3A2 可抑制 NB 细胞的生长。为了研究 SLC3A2 靶向的治疗潜力,我们用 AMXT-1501(一种多胺转运抑制剂)对 NB 细胞进行了单药处理,结果显示 AMXT-1501 对 NB 细胞的作用一般。与此相反,洛拉替尼/AMXT-1501联合治疗可协同抑制ALK驱动的NB细胞系的细胞生长。综上所述,我们的研究结果确定了ALK受体酪氨酸激酶(RTK)与MARCH11 E3连接酶协同调节NB细胞中SLC3A2蛋白稳定性和功能的新作用。联合抑制ALK和多胺转运的协同效应表明,ALK/MARCH11/SLC3A2对氨基酸转运的调控对NB细胞的致癌生长和存活非常重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


