Human adipose-derived stem cells genetically programmed to induce necroptosis for cancer immunotherapy
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Herein, we present human adipose-derived stem cells (ADSCs) inserted with the receptor-interacting protein kinase-3 (RIP3) gene (RP@ADSCs), which induces cell necroptosis, for tumor immunotherapy. Necroptosis has characteristics of both apoptosis, such as programmed cell death, and necrosis, such as swelling and plasma membrane rupture, during which damage-related molecular patterns are released, triggering an immune response. Therefore, necroptosis has the potential to be used as an effective anticancer immunotherapy. RP@ADSCs were programmed to necroptosis after a particular time after being injected in vivo, and various pro-inflammatory cytokines secreted during the stem cell death process stimulated the immune system, showing local and sustained anticancer effects. It was confirmed that RIP3 protein expression increased in ADSCs after RP transfection. RP@ADSCs continued to induce ADSCs death for 7 days, and various pro-inflammatory cytokines were secreted through ADSCs death. The efficacy of RP@ADSCs-mediated immunotherapy was evaluated in mouse models bearing GL-26 (glioblastoma) and K1735 (melanoma), and it was found that RP resulted in an increase in the population of long-term cytotoxic T cells and a decrease in the population of regulatory T cells. This shows that RP@ADSCs have potential and applicability as an excellent anticancer immunotherapy agent in clinical practice.
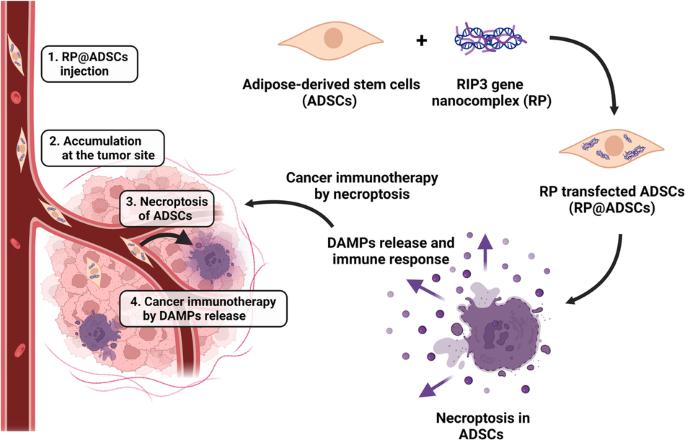
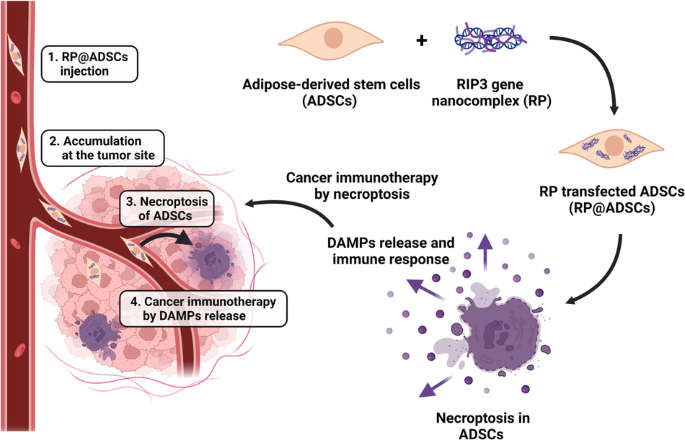
通过基因编程诱导坏死的人类脂肪源性干细胞用于癌症免疫疗法。
在本文中,我们介绍了插入受体相互作用蛋白激酶-3(RIP3)基因(RP@ADSCs)的人脂肪来源干细胞(ADSCs),该基因可诱导细胞坏死,用于肿瘤免疫疗法。坏死既有细胞凋亡(如程序性细胞死亡)的特征,也有坏死(如肿胀和质膜破裂)的特征,在坏死过程中会释放与损伤相关的分子模式,从而引发免疫反应。因此,坏死有可能被用作一种有效的抗癌免疫疗法。RP@ADSCs经体内注射后在特定时间内发生坏死,干细胞坏死过程中分泌的各种促炎细胞因子刺激了免疫系统,显示出局部和持续的抗癌作用。研究证实,RP转染后,ADSCs中RIP3蛋白表达增加。RP@ADSCs可持续诱导ADSCs死亡7天,ADSCs死亡过程中会分泌多种促炎细胞因子。研究人员在GL-26(胶质母细胞瘤)和K1735(黑色素瘤)小鼠模型中评估了RP@ADSCs介导的免疫疗法的疗效,结果发现RP导致长期细胞毒性T细胞数量增加,调节性T细胞数量减少。这表明,RP@ADSCs 作为一种优秀的抗癌免疫疗法制剂在临床实践中具有潜力和适用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


