Baseline neurological deficit and argatroban plus alteplase in acute ischemic stroke: A post hoc analysis of ARAIS trial
IF 5.6
2区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
Background
The ARAIS trial didn’t demonstrate argatroban significantly improve functional outcome at 90 days in acute ischemic stroke. We conducted post hoc analysis of ARAIS to investigate whether baseline neurological deficit was associated with outcomes.
Methods
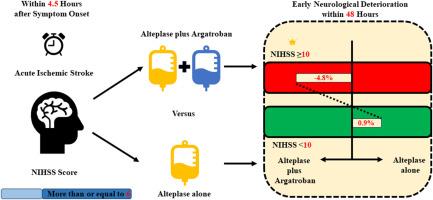
Patients without endovascular therapy who met screening criteria as protocol and completed argatroban treatment were enrolled and classified into two subgroups according to NIHSS score at admission. Primary outcome was excellent functional outcome at 90 days, defined as mRS score of 0 to 1. Early neurological deterioration (END), defined as an increase of ≥4 in the NIHSS score from baseline within 48 hours, was investigated as secondary outcome. Compared with alteplase alone, we investigated treatment effect of argatroban plus alteplase on outcomes in subgroups and interaction with subgroups.
Results
A total of 675 patients from full analysis set were included: 390 were assigned into NIHSS score <10 subgroup and 285 into NIHSS score ≥10 subgroup. For primary outcome, there was similar treatment effect between argatroban plus alteplase and alteplase alone in NIHSS score ≥10 subgroup (adjusted RD, 5.8%; 95% CI, −6.0% to 17.5%; P = 0.33) and in NIHSS score <10 subgroup (adjusted RD, −1.4%; 95% CI, −9.9% to 7.1%; P = 0.75), and no significant interaction (P = 0.43). Occurrence of early neurological deterioration within 48 hours were significantly lower in NIHSS score ≥10 subgroup, compared with NIHSS score <10 subgroup (P = 0.006).
Conclusion
Among patients with NIHSS score ≥10, argatroban plus alteplase could safely reduce END within 48 hours.
阿加曲班加阿替普酶治疗急性缺血性脑卒中的基线神经功能缺损:ARAIS试验的事后分析。
背景:ARAIS试验并未证明阿加曲班能显著改善急性缺血性卒中患者90天后的功能预后。我们对 ARAIS 进行了事后分析,研究基线神经功能缺损是否与预后相关:方法:我们纳入了未接受血管内治疗、符合方案筛选标准并完成阿加曲班治疗的患者,并根据入院时的 NIHSS 评分将其分为两个亚组。主要结果是 90 天后的良好功能预后,即 mRS 评分为 0 至 1 分。早期神经功能恶化(END)是指在48小时内NIHSS评分比基线增加≥4分,它是次要结果。与单用阿替普酶相比,我们研究了阿加曲班加阿替普酶对亚组结局的治疗效果以及与亚组的交互作用:共有 675 名患者被纳入完整分析集:390例被归入NIHSS评分结论:在NIHSS评分≥10分的患者中,阿加曲班加阿替普酶可在48小时内安全地减少END。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neurotherapeutics
医学-神经科学
CiteScore
11.00
自引率
3.50%
发文量
154
审稿时长
6-12 weeks
期刊介绍:
Neurotherapeutics® is the journal of the American Society for Experimental Neurotherapeutics (ASENT). Each issue provides critical reviews of an important topic relating to the treatment of neurological disorders written by international authorities.
The Journal also publishes original research articles in translational neuroscience including descriptions of cutting edge therapies that cross disciplinary lines and represent important contributions to neurotherapeutics for medical practitioners and other researchers in the field.
Neurotherapeutics ® delivers a multidisciplinary perspective on the frontiers of translational neuroscience, provides perspectives on current research and practice, and covers social and ethical as well as scientific issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


