Chaperone- and PTM-mediated activation of IRF1 tames radiation-induced cell death and the inflammatory response
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
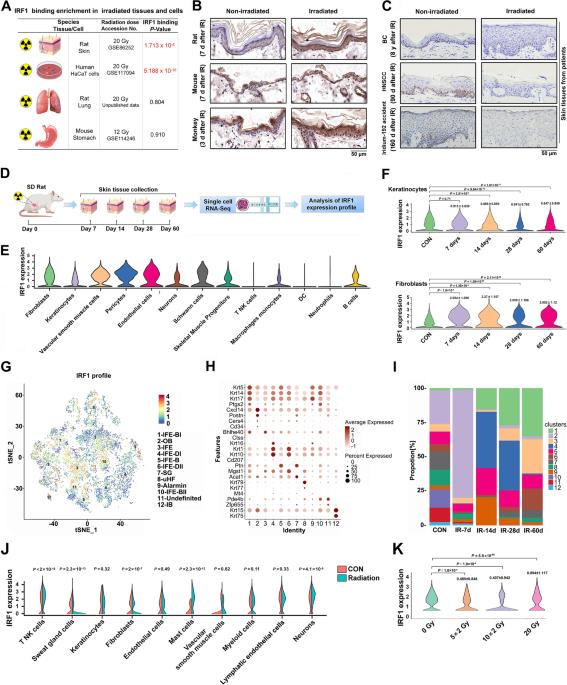
The key role of structural cells in immune modulation has been revealed with the advent of single-cell multiomics, but the underlying mechanism remains poorly understood. Here, we revealed that the transcriptional activation of interferon regulatory factor 1 (IRF1) in response to ionizing radiation, cytotoxic chemicals and SARS-CoV-2 viral infection determines the fate of structural cells and regulates communication between structural and immune cells. Radiation-induced leakage of mtDNA initiates the nuclear translocation of IRF1, enabling it to regulate the transcription of inflammation- and cell death-related genes. Novel posttranslational modification (PTM) sites in the nuclear localization sequence (NLS) of IRF1 were identified. Functional analysis revealed that mutation of the acetylation site and the phosphorylation sites in the NLS blocked the transcriptional activation of IRF1 and reduced cell death in response to ionizing radiation. Mechanistically, reciprocal regulation between the single-stranded DNA sensors SSBP1 and IRF1, which restrains radiation-induced and STING/p300-mediated PTMs of IRF1, was revealed. In addition, genetic deletion or pharmacological inhibition of IRF1 tempered radiation-induced inflammatory cell death, and radiation mitigators also suppressed SARS-CoV-2 NSP-10-mediated activation of IRF1. Thus, we revealed a novel cytoplasm-oriented mechanism of IRF1 activation in structural cells that promotes inflammation and highlighted the potential effectiveness of IRF1 inhibitors against immune disorders.
伴侣和 PTM 介导的 IRF1 激活可控制辐射诱导的细胞死亡和炎症反应。
单细胞多组学的出现揭示了结构细胞在免疫调节中的关键作用,但对其潜在机制仍知之甚少。在这里,我们揭示了干扰素调节因子1(IRF1)在电离辐射、细胞毒性化学物质和SARS-CoV-2病毒感染下的转录激活决定了结构细胞的命运,并调节结构细胞和免疫细胞之间的交流。辐射诱导的 mtDNA 泄漏启动了 IRF1 的核转位,使其能够调节炎症和细胞死亡相关基因的转录。研究人员在IRF1的核定位序列(NLS)中发现了新的翻译后修饰(PTM)位点。功能分析显示,NLS中乙酰化位点和磷酸化位点的突变阻断了IRF1的转录激活,并减少了电离辐射下的细胞死亡。从机理上讲,单链DNA传感器SSBP1和IRF1之间存在相互调控,抑制了辐射诱导和STING/p300介导的IRF1的PTMs。此外,基因缺失或药物抑制 IRF1 可抑制辐射诱导的炎症细胞死亡,辐射缓解剂也可抑制 SARS-CoV-2 NSP-10 介导的 IRF1 激活。因此,我们揭示了IRF1在结构细胞中促进炎症的一种新的面向细胞质的活化机制,并强调了IRF1抑制剂对免疫紊乱的潜在功效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


