Determination of N-centered stereochemistry in N22-methylated chlorophyll-a derivatives and their epimer-dependent optical spectra
IF 3
4区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
An N-centered epimeric mixture of chlorophyll-a derivatives methylated at the inner nitrogen atom was separated by reverse-phase high-performance liquid chromatography. Circular dichroism (CD) spectroscopic analyses of the epimerically pure N22-methyl-chlorins revealed that the minor first-eluted and major second-eluted stereoisomers were (22S)- and (22R)-configurations, respectively. Their visible absorption and CD spectra in solution were dependent on the N22-stereochemistry. The epimer-dependent spectral changes were independent of the substituents at the peripheral 3-position of the core chlorin chromophore.
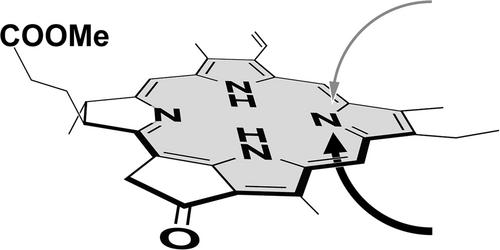
确定 N22-甲基化叶绿素-a 衍生物中的 N 中心立体化学及其表聚体依赖性光学光谱。
通过反相高效液相色谱法分离了内氮原子甲基化的叶绿素-a 衍生物的 N-中心二元混合物。对N22-甲基-氯素的二价纯化物进行的圆二色性(CD)光谱分析显示,第一次洗脱的次要立体异构体和第二次洗脱的主要立体异构体分别为(22S)-和(22R)-构型。它们在溶液中的可见吸收光谱和 CD 光谱取决于 N22 立体化学结构。依赖于外延体的光谱变化与核心氯素发色团外围 3 位的取代基无关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Chirality
医学-分析化学
CiteScore
4.40
自引率
5.00%
发文量
124
审稿时长
1 months
期刊介绍:
The main aim of the journal is to publish original contributions of scientific work on the role of chirality in chemistry and biochemistry in respect to biological, chemical, materials, pharmacological, spectroscopic and physical properties.
Papers on the chemistry (physiochemical, preparative synthetic, and analytical), physics, pharmacology, clinical pharmacology, toxicology, and other biological aspects of chiral molecules will be published.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


