Positive allosteric modulation of the cannabinoid CB1 receptor potentiates endocannabinoid signalling and changes ERK1/2 phosphorylation kinetics
Abstract
Background and Purpose
Activation of CB1 by exogenous agonists causes adverse effects in vivo. Positive allosteric modulation may offer improved therapeutic potential and a reduced on-target adverse effect profile compared with orthosteric agonists, due to reduced desensitisation/tolerance, but this has not been directly tested. This study investigated the ability of PAMs/ago-PAMs to induce receptor regulation pathways, including desensitisation and receptor internalisation.
Experimental Approach
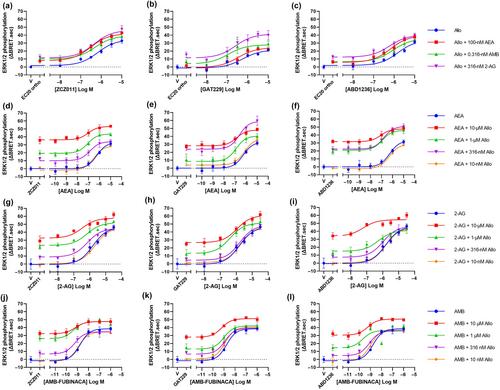
Bioluminescence resonance energy transfer (BRET) assays in HEK293 cells were performed to investigate G protein dissociation, ERK1/2 phosphorylation and β-arrestin 2 translocation, while immunocytochemistry was performed to measure internalisation of CB1 in response to the PAMs ZCZ011, GAT229 and ABD1236 alone and in combination with the orthosteric agonists AEA, 2-AG, and AMB-FUBINACA.
Key Results
ZCZ011, GAT229 and ABD1236 were allosteric agonists in all pathways tested. The ago-PAM ZCZ011 induced a biphasic ERK1/2 phosphorylation time course compared to transient activation by orthosteric agonists. In combination with 2-AG but not AEA or AMB-FUBINACA, ZCZ011 and ABD1236 caused the transient peak of ERK1/2 phosphorylation to become sustained. All PAMs increased the potency and efficacy of AEA-induced signalling in all pathways tested; however, no notable potentiation of 2-AG or AMB-FUBINACA was observed.
Conclusion and Implications
Ago-PAMs can potentiate endocannabinoid CB1 agonism by AEA to a larger extent compared with 2-AG. However, all compounds were found to be allosteric agonists and induce activation of CB1 in the absence of endocannabinoid, including β-arrestin 2 recruitment and internalisation. Thus, the spatiotemporal signalling of endogenous cannabinoids will not be retained in vivo.
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


