TRIM33 plays a critical role in regulating dendritic cell differentiation and homeostasis by modulating Irf8 and Bcl2l11 transcription
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
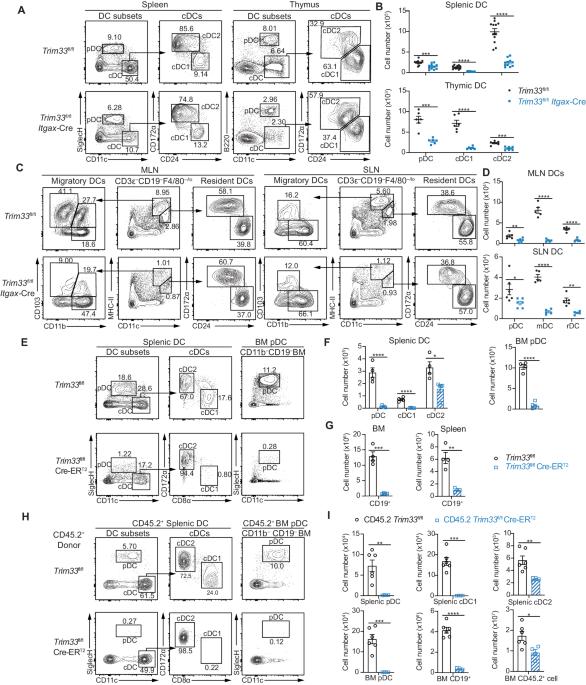
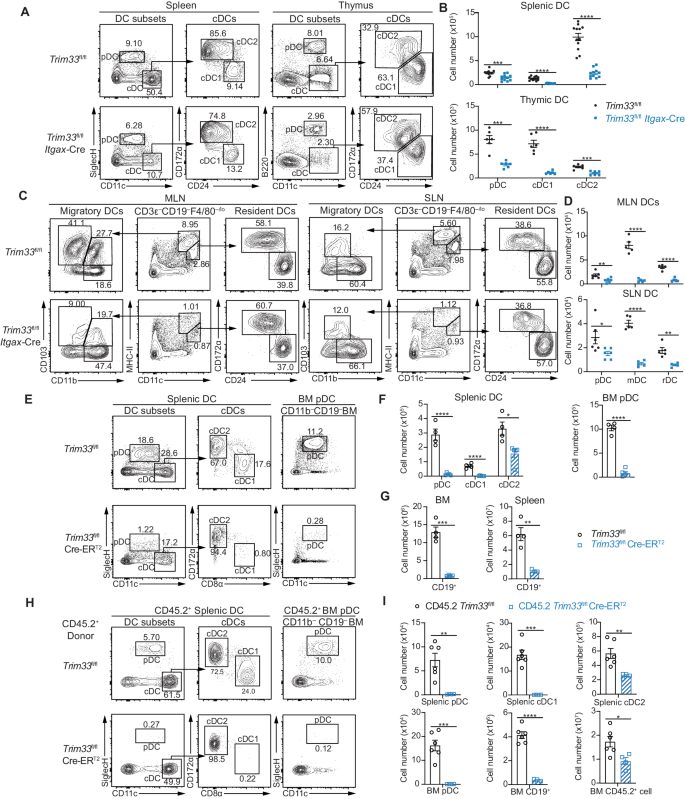
The development of distinct dendritic cell (DC) subsets, namely, plasmacytoid DCs (pDCs) and conventional DC subsets (cDC1s and cDC2s), is controlled by specific transcription factors. IRF8 is essential for the fate specification of cDC1s. However, how the expression of Irf8 is regulated is not fully understood. In this study, we identified TRIM33 as a critical regulator of DC differentiation and maintenance. TRIM33 deletion in Trim33fl/fl Cre-ERT2 mice significantly impaired DC differentiation from hematopoietic progenitors at different developmental stages. TRIM33 deficiency downregulated the expression of multiple genes associated with DC differentiation in these progenitors. TRIM33 promoted the transcription of Irf8 to facilitate the differentiation of cDC1s by maintaining adequate CDK9 and Ser2 phosphorylated RNA polymerase II (S2 Pol II) levels at Irf8 gene sites. Moreover, TRIM33 prevented the apoptosis of DCs and progenitors by directly suppressing the PU.1-mediated transcription of Bcl2l11, thereby maintaining DC homeostasis. Taken together, our findings identified TRIM33 as a novel and crucial regulator of DC differentiation and maintenance through the modulation of Irf8 and Bcl2l11 expression. The finding that TRIM33 functions as a critical regulator of both DC differentiation and survival provides potential benefits for devising DC-based immune interventions and therapies.
TRIM33 通过调节 Irf8 和 Bcl2l11 的转录,在树突状细胞分化和稳态中发挥着关键作用。
不同树突状细胞(DC)亚群,即质体 DC(pDCs)和传统 DC 亚群(cDC1s 和 cDC2s)的发育受特定转录因子的控制。IRF8对cDC1s的命运分化至关重要。然而,Irf8 的表达是如何被调控的还不完全清楚。在这项研究中,我们发现 TRIM33 是 DC 分化和维持的关键调控因子。在Trim33fl/fl Cre-ERT2小鼠中缺失TRIM33会显著影响造血祖细胞在不同发育阶段的DC分化。TRIM33的缺失下调了这些祖细胞中与DC分化相关的多个基因的表达。TRIM33 通过在 Irf8 基因位点维持足够的 CDK9 和 Ser2 磷酸化 RNA 聚合酶 II(S2 Pol II)水平,促进 Irf8 的转录,从而促进 cDC1s 的分化。此外,TRIM33 通过直接抑制 PU.1 介导的 Bcl2l11 转录,防止了 DCs 和祖细胞的凋亡,从而维持了 DC 的稳态。综上所述,我们的研究结果表明,TRIM33 是通过调节 Irf8 和 Bcl2l11 的表达来调节直流分化和维持的一种新的关键调控因子。TRIM33是直流分化和存活的关键调节因子,这一发现为设计基于直流的免疫干预和疗法提供了潜在的益处。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


