Sulthiame use in children with pharmacoresistant epilepsies: A retrospective study
Abstract
Objective
This retrospective study aimed to assess the efficacy and tolerability of sulthiame as an add-on treatment in children with pharmacoresistant epilepsies.
Methods
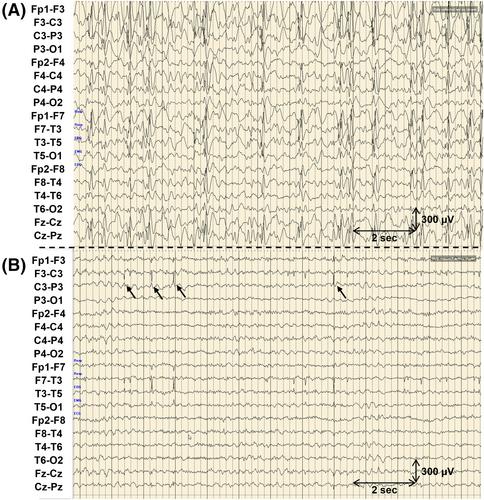
All patients with epilepsy who received sulthiame at Montreal Children's Hospital over an 11-year period were included. Medical charts were reviewed, and extracted data included patient age and sex, seizure types, epilepsy syndrome, electroencephalography (EEG) reports, brain imaging reports, antiseizure treatments trialed, starting and final dose of sulthiame, duration of sulthiame treatment, adverse events attributed to sulthiame, and seizure frequency before and after sulthiame treatment. EEG studies were also analyzed and spike–wave index (SWI) in the first 10 min of sleep was calculated.
Results
Sixteen patients were included, all of whom had pharmacoresistant epilepsies (mean of 9.9 trials of other antiseizure treatments). Six had genetic diagnoses, four had in utero/perinatal acquired brain injury, one had a suspected focal cortical dysplasia, and five were idiopathic. Ten patients had developmental and epileptic encephalopathy with spike–wave activation in sleep, three had Lennox–Gastaut syndrome, and one each had sleep-related hyperkinetic epilepsy, self-limited epilepsy with centrotemporal spikes, and mixed generalized and multifocal epilepsy. Of the 12 patients with uncontrolled seizures at the time of sulthiame initiation, 4 had improvement in seizure frequency, including 2 who became seizure free. Eight patients had EEG data available that allowed calculation of sleep SWI; from this group, SWI decreased from 81.1% +/− 17.6% to 45.1% +/− 36.5% (p = .007). The most common adverse events reported were somnolence/drowsiness, aggression, and increased seizure frequency. Of the patients with genetic etiologies, significant positive responses were seen in patients with pathogenic variants in NDUFS1 and SATB1.
Significance
These data demonstrate the therapeutic potential of sulthiame, even in patients with highly pharmacoresistant epilepsy. Improvements may be seen in both seizure frequency and sleep SWI.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


