BRD9 regulates normal human hematopoietic stem cell function and lineage differentiation
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
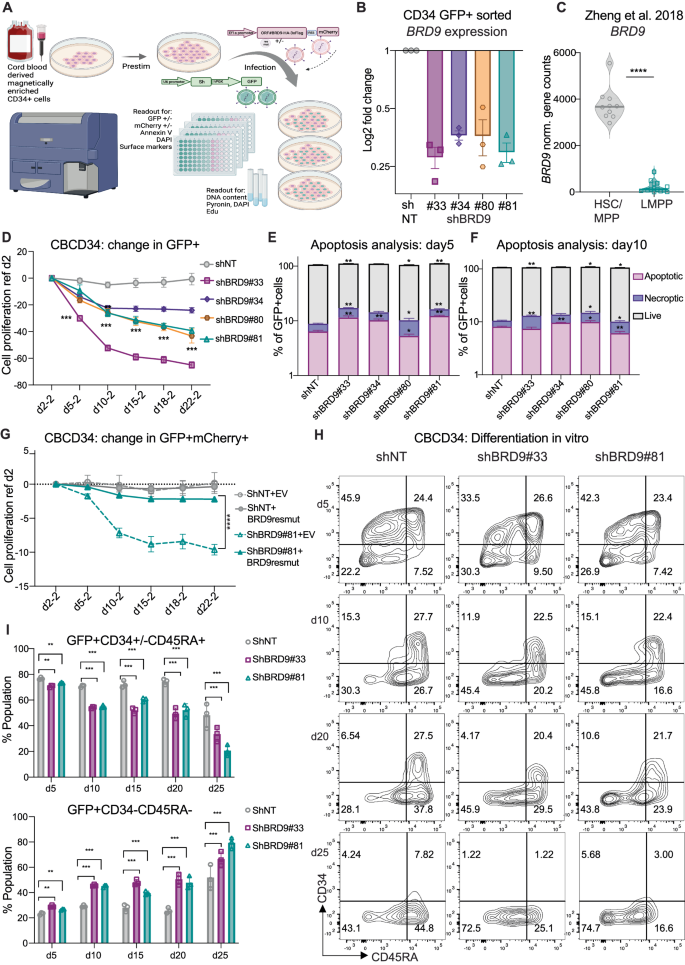
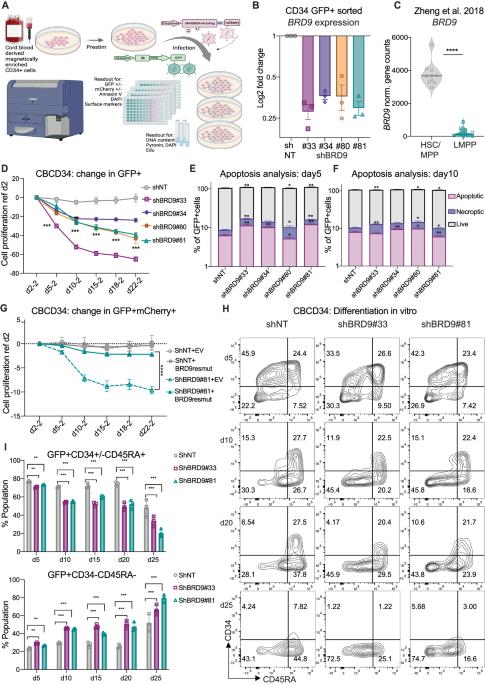
Bromodomain containing protein 9 (BRD9), a member of the non-canonical BRG1/BRM-associated factor (ncBAF) chromatin remodeling complex, has been implicated as a synthetic lethal target in AML but its function in normal human hematopoiesis is unknown. In hematopoietic stem and progenitor cells (HSPC) genomic or chemical inhibition of BRD9 led to a proliferative disadvantage and loss of stem cells in vitro. Human HSPCs with reduced BRD9 protein levels produced lower numbers of immature mixed multipotent GEMM colonies in semi-solid media. In lineage-promoting culture conditions, cells with reduced BRD9 levels failed to differentiate into the megakaryocytic lineage and showed delayed differentiation into erythroid cells but enhanced terminal myeloid differentiation. HSPCs with BRD9 knock down (KD) had reduced long-term multilineage engraftment in a xenotransplantation assay. An increased number of downregulated genes in RNAseq analysis after BRD9 KD coupled with a gain in chromatin accessibility at the promoters of several repressive transcription factors (TF) suggest that BRD9 functions in the maintenance of active transcription during HSC differentiation. In particular, the hematopoietic master regulator GATA1 was identified as one of the core TFs regulating the gene networks modulated by BRD9 loss in HSPCs. BRD9 inhibition reduced a GATA1-luciferase reporter signal, further suggesting a role for BRD9 in regulating GATA1 activity. BRD9 is therefore an additional example of epigenetic regulation of human hematopoiesis.
BRD9调控正常人类造血干细胞的功能和品系分化
含溴结构域蛋白9(BRD9)是非典型BRG1/BRM相关因子(ncBAF)染色质重塑复合物的成员,已被认为是急性髓细胞性白血病的合成致死靶点,但其在正常人类造血过程中的功能尚不清楚。在造血干细胞和祖细胞(HSPC)中,对BRD9进行基因组或化学抑制会导致体外干细胞的增殖劣势和丧失。BRD9蛋白水平降低的人类HSPC在半固体培养基中产生的未成熟混合多能GEMM集落数量较少。在品系促进培养条件下,BRD9水平降低的细胞无法向巨核细胞品系分化,并表现出向红细胞分化延迟,但末端髓系分化增强。在异种移植试验中,BRD9基因敲除(KD)的HSPC长期多系移植能力下降。BRD9 KD后,RNAseq分析中下调基因的数量增加,再加上一些抑制性转录因子(TF)启动子染色质可及性的增加,表明BRD9在造血干细胞分化过程中具有维持活性转录的功能。特别是,造血主调节因子 GATA1 被确定为调节 HSPCs 中受 BRD9 缺失影响的基因网络的核心 TF 之一。抑制BRD9可减少GATA1-荧光素酶报告信号,这进一步表明BRD9在调节GATA1活性中的作用。因此,BRD9是人类造血过程中表观遗传调控的又一个实例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


