Transcriptomics of differences in thermal plasticity associated with selection for an exaggerated male sexual trait
IF 3.9
2区 生物学
Q2 ECOLOGY
引用次数: 0
Abstract
The information about the magnitude of differences in thermal plasticity both between and within populations, as well as identification of the underlying molecular mechanisms are key to understanding the evolution of thermal plasticity. In particular, genes underlying variation in the physiological response to temperature can provide raw material for selection acting on plastic traits. Using RNAseq, we investigate the transcriptional response to temperature in males and females from bulb mite populations selected for the increased frequency of one of two discrete male morphs (fighter- and scrambler-selected populations) that differ in relative fitness depending on temperature. We show that different mechanisms underlie the divergence in thermal response between fighter- and scrambler-selected populations at decreased vs. increased temperature. Temperature decrease to 18 °C was associated with higher transcriptomic plasticity of males with more elaborate armaments, as indicated by a significant selection-by-temperature interaction effect on the expression of 40 genes, 38 of which were upregulated in fighter-selected populations in response to temperature decrease. In response to 28 °C, no selection-by-temperature interaction in gene expression was detected. Hence, differences in phenotypic response to temperature increase likely depended on genes associated with their distinct morph-specific thermal tolerance. Selection of males also drove gene expression patterns in females. These patterns could be associated with temperature-dependent fitness differences between females from fighter- vs. scrambler-selected populations reported in previous studies. Our study shows that selection for divergent male sexually selected morphologies and behaviors has a potential to drive divergence in metabolic pathways underlying plastic response to temperature in both sexes.
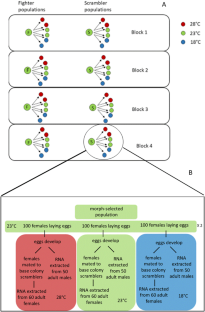
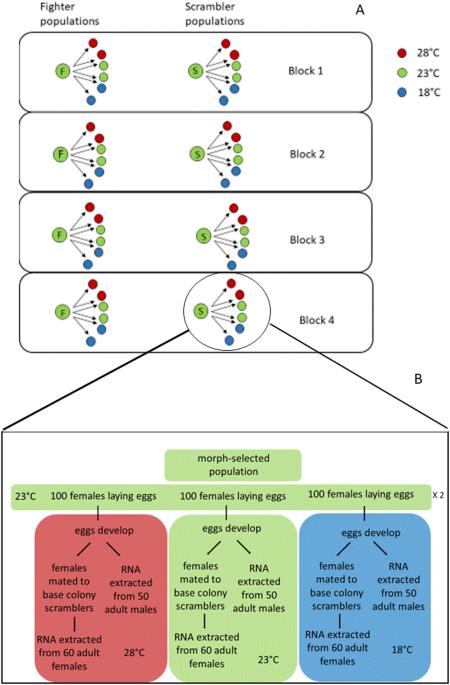
热可塑性差异的转录组学与雄性性状选择有关。
关于种群之间和种群内部热可塑性差异大小的信息,以及对其分子机制的鉴定,是了解热可塑性进化的关键。特别是,温度生理反应变异的基础基因可以为可塑性特征的选择提供原始材料。利用 RNAseq,我们研究了球螨种群中雄性和雌性对温度的转录反应,这些种群被选育为两种离散雄性形态(战斗者和扰乱者选育种群)之一,它们的相对适合度随温度而不同。我们的研究表明,在温度降低与升高的情况下,战斗者和扰乱者选择种群的热反应差异是由不同的机制造成的。温度降低到18 °C时,雄性战斗机种群的转录组具有更高的可塑性,这表现在40个基因的表达具有显著的选择-温度交互效应,其中38个基因在温度降低时上调。在对 28 ℃ 的反应中,没有发现基因表达在不同温度下的选择相互作用。因此,对温度升高的表型反应差异可能取决于与不同形态特异性耐热性相关的基因。雄性的选择也会影响雌性的基因表达模式。这些模式可能与以往研究中报道的战斗者与扰乱者选择种群中雌性的适应性差异有关。我们的研究表明,对雄性性选择的不同形态和行为的选择有可能驱动两性对温度的可塑性反应的代谢途径的差异。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Heredity
生物-进化生物学
CiteScore
7.50
自引率
2.60%
发文量
84
审稿时长
4-8 weeks
期刊介绍:
Heredity is the official journal of the Genetics Society. It covers a broad range of topics within the field of genetics and therefore papers must address conceptual or applied issues of interest to the journal''s wide readership
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


