Molecular Basis of Absorption at 340 nm of 3-Ketoglucosides under Alkaline Conditions.
IF 1.4
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Journal of applied glycoscience
Pub Date : 2024-04-20
eCollection Date: 2024-01-01
DOI:10.5458/jag.jag.JAG-2023_0014
引用次数: 0
Abstract
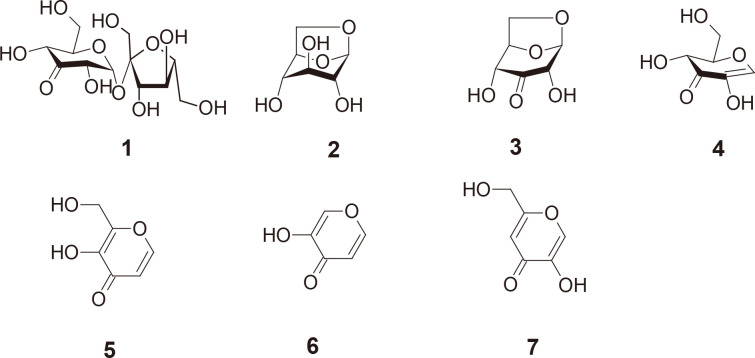
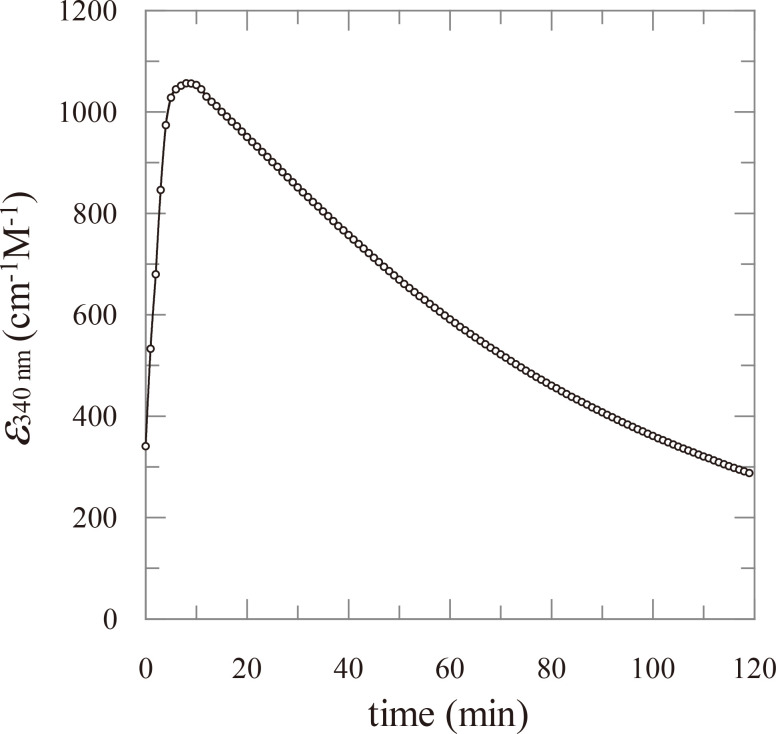
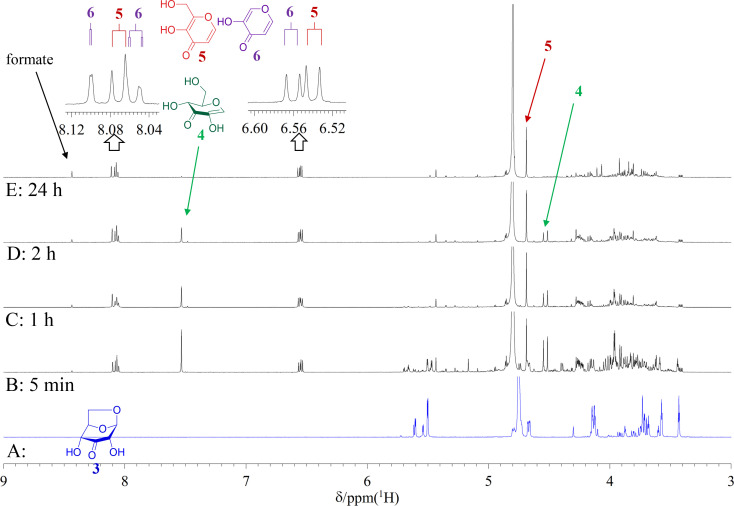
Transient absorption at 340 nm under alkaline conditions has long been used to detect the presence of 3-keto-O-glycosides without understanding the molecular basis of the absorbance. The time course of A340 nm for the alkaline treatment of 3-ketolevoglucosan, an intramolecular 3-keto-O-glycoside, was investigated to identify the three products generated through alkaline treatment. By comparing the spectra of these compounds under neutral and alkaline conditions, we identified 1,5-anhydro-D-erythro-hex-1-en-3-ulose (2-hydroxy-3-keto-D-glucal) as being the compound responsible for the absorption.
碱性条件下 3-Ketoglucosides 在 340 纳米波长处吸收的分子基础。
长期以来,在碱性条件下 340 纳米波长处的瞬态吸收一直被用来检测 3-酮-O-糖苷的存在,但却不了解吸光度的分子基础。我们研究了分子内 3-酮-O-糖苷在碱性处理 3-ketolevoglucosan 时 A340 nm 波长的时间过程,以确定碱性处理产生的三种产物。通过比较这些化合物在中性和碱性条件下的光谱,我们确定 1,5-anhydro-D-erythro-hex-1-en-3-ulose (2-hydroxy-3-keto-D-glucal) 是导致吸收的化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


