Synthesis of Dihydrobenzosiloles and Silacyclopentane via Double Hydroalumination of Terminal Alkynes
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We developed an efficient method for the synthesis of dihydrobenzosiloles in 19% to 90% isolated yields from 1-hydrosilyl-2-ethynylbenzenes using two equivalents of diisobutylaluminum hydride. The reaction mechanism involves regioselective double hydroalumination of the alkyne moiety followed by cyclization to 2-alanyldihydrobenzosilole. A silacyclopentane was also synthesized in 97% isolated yield from the corresponding 4-silylbut-1-yne under the same reaction conditions. Although the substrate scope was conducted on a 0.5 mmol scale, a gram-scale reaction of 1-diphenylsilyl-2-ethynylbenzene under the optimized reaction conditions successfully afforded the desired product in 94% isolated yield without loss of reactivity.
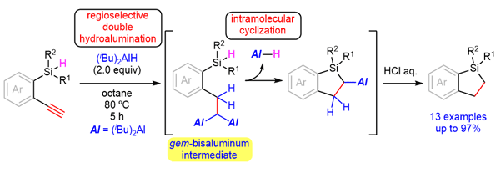
通过末端炔烃的双氢化氨合成二氢苯甲硅烷和硅杂环戊烷
我们开发了一种高效的方法,利用两个当量的二异丁基氢化铝,从 1- 氢硅烷基-2-乙炔基苯合成二氢苯甲硅烷基,分离产率为 19% 至 90%。反应机理包括炔基的区域选择性双氢化,然后环化成 2-丙烯基二氢苯甲硅醚。在相同的反应条件下,还从相应的 4-硅基丁基-1-炔合成出了一种硅基环戊烷,分离收率为 97%。虽然底物范围是在 0.5 毫摩尔的规模下进行的,但在优化的反应条件下,1-二苯基硅基-2-乙炔基苯的克级反应成功地得到了所需的产物,分离收率为 94%,且没有失去反应活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


