Copper−Promoted Dimerization of Benzyl Thiocyanates to Access Functionalized Bibenzyls
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The synthesis of bibenzyl derivatives holds significance in organic chemistry due to their diverse pharmacological and synthetic applications. Herein, we report a novel copper–promoted dimerization reaction for the efficient synthesis of functionalized bibenzyls from benzyl thiocyanates. The coupling reaction proceeds under aerobic and mild conditions via cascade C–S bond cleavage in one pot. Diverse substituents including electron–withdrawing groups on the aryl ring are well–tolerated to afford the desired products in moderate to good yields. The developed protocol could be utilized to obtain the cross coupling product from two different electron–deficient benzyl thiocyanates.
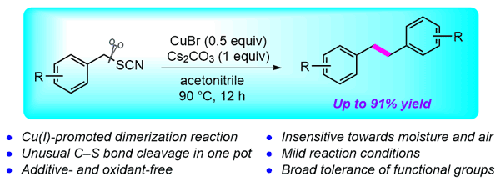
铜促进硫氰酸苄酯二聚化以获得官能化的比本苄
由于联苄衍生物具有多种药理和合成应用,因此其合成在有机化学中具有重要意义。在此,我们报告了一种新型的铜促进二聚反应,用于从硫氰酸苄酯高效合成功能化的联苄。该偶联反应在有氧和温和的条件下通过级联 C-S 键裂解在一锅中进行。芳基环上的各种取代基(包括抽电子基团)都能很好地耐受,从而以中等至较高的产率得到所需的产物。所开发的方案可用于从两种不同的缺电子硫氰酸苄酯中获得交叉偶联产物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


