Structural simplification and ester bond flipping lead to bis-benzodioxole derivatives as potential hypolipidemic and hepatoprotective agent
Abstract
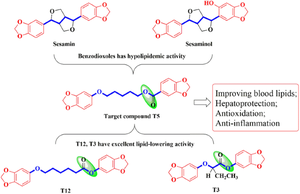
A series of bis-benzodioxole derivatives was designed, synthesized, and evaluated. These target compounds were designed through structure simplification and ester bond flipping. The lipid-lowering activity of these target compounds was preliminarily evaluated in a hyperlipidemic mouse model induced by Triton WR 1339. The results showed that piperonylic acid -6-(3,4-methylenedioxyphenoxy) hexyl ester (T5) possesses notable lipid-lowering properties, reducing triglyceride (TG) and total cholesterol (TC) levels. The dose-dependent study revealed that compound T5 decreased TG and TC more strongly with the increase of dose. It was observed that T5 had considerable effects on decreasing TG, TC and low density lipoprotein cholesterol (LDL-C) levels in hyperlipidemic mice induced by high fat diet (HFD). Meanwhile, T5 was found to have hepatoprotective activity, with the liver aspartate transaminase (AST) and alanine aminotransferase (ALT) significantly decreasing and histopathological observation showing that it inhibited lipids accumulation in the liver and alleviated liver injury. T5 has been shown to stimulate peroxisome proliferator-activated receptor-α (PPAR-α) and suppress hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase in the liver in connection to lipid metabolism. The molecular docking study also revealed that T5 has a high affinity for the active sites of PPAR-α and HMG-CoA reductase. Furthermore, other beneficial activities, including antioxidation and anti-inflammation, were also noted. It is possible that further exploration may result in compound T5 becoming a promising candidate for lipid-lowering therapy.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


