Simple and green synthesis of 3-acyl-pyrazolo[1,5-a]pyridines: [3+2] through cycloaddition of N-aminopyridines on enaminones
IF 2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A practicable method is described for the synthesis of 3-acyl-pyrazolo[1,5-a]pyridines from N-aminopyridines and easily available enaminones via a [3+2] cycloaddition. The reactions proceed well with a broad scope, without using additive (base or oxidant).
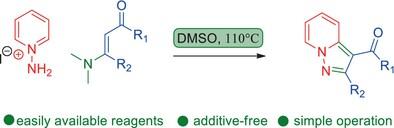

3-acyl-pyrazolo[1,5-a]pyridines 的简单绿色合成:通过 N-氨基吡啶与烯胺酮的环加成反应实现 [3+2]
本文介绍了一种通过 [3+2] 环加成法从 N-氨基吡啶和容易获得的烯酰胺酮合成 3-酰基吡唑并[1,5-a]吡啶的实用方法。反应进行顺利,范围广泛,无需使用添加剂(碱或氧化剂)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.20
自引率
4.20%
发文量
177
审稿时长
3.9 months
期刊介绍:
The Journal of Heterocyclic Chemistry is interested in publishing research on all aspects of heterocyclic chemistry, especially development and application of efficient synthetic methodologies and strategies for the synthesis of various heterocyclic compounds. In addition, Journal of Heterocyclic Chemistry promotes research in other areas that contribute to heterocyclic synthesis/application, such as synthesis design, reaction techniques, flow chemistry and continuous processing, multiphase catalysis, green chemistry, catalyst immobilization and recycling.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


