Enhancing Electrochemical Efficiency of Solid Oxide Electrolysis Cells for Carbon Dioxide Reduction Through Nickel‐Doped Titanate‐Based Cathode with Doped Ceria Electrolyte
引用次数: 0
Abstract
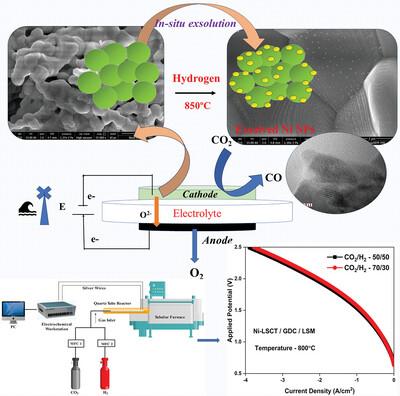
Solid oxide electrolysis cell (SOEC) is a potential technology for converting the principal greenhouse gas, carbon dioxide (CO2), into carbon monoxide (CO) by employing renewable energy. SOECs have great potential, including high‐energy efficiency, fast electrode kinetics, and competitive cost; however, this technology still has challenges in developing highly active, robust CO2 cathode electrocatalysts. In this work, we report the Ni‐doped lanthanum strontium calcium titanate (La0.20Sr0.25Ca0.45Ni0.05Ti0.95O3−δ) cathode for application as the cathode of CO2 electrolysis with gadolinia‐doped ceria (Gd0.1Ce0.9O1.95) electrolyte in SOEC. The exsolution of Ni nanoparticles is achieved by a simple in situ growth method at 800 °C. The Ni doping in LSCT significantly improved the electrochemical activity of the catalyst by increasing oxygen vacancies, and the Ni metallic nanoparticles can afford much more active sites for CO2 reduction. The CO2 electrolysis mechanism is studied by the distribution of relaxation time analysis of impedance spectroscopy. Ni‐LSCT renders a higher activity for electrolysis of CO2 with an exceptionally high reduction current density of 3.89 A cm−2 at 2.5 V potential applied and 800 °C temperature with GDC (Gd0.1Ce0.9O1.95) electrolyte. Ni doping is a crucial factor in controlling the electrochemical performance and catalytic activity in SOEC and GDC electrolytes, which is further helped by the high ionic conductivity.
通过掺杂铈电解质的掺镍钛酸基阴极提高用于二氧化碳还原的固体氧化物电解池的电化学效率
固体氧化物电解池(SOEC)是一种利用可再生能源将主要温室气体二氧化碳(CO2)转化为一氧化碳(CO)的潜在技术。SOEC 具有巨大的潜力,包括高能效、快速电极动力学和具有竞争力的成本;然而,该技术在开发高活性、坚固的 CO2 阴极电催化剂方面仍面临挑战。在这项工作中,我们报告了掺杂镍的钛酸镧锶钙盐(La0.20Sr0.25Ca0.45Ni0.05Ti0.95O3-δ)阴极在 SOEC 中用作掺杂钆的陶瓷(Gd0.1Ce0.9O1.95)电解质的二氧化碳电解阴极。镍纳米颗粒的溶解是通过一种简单的原位生长方法在 800 °C 下实现的。通过增加氧空位,LSCT 中的掺杂镍大大提高了催化剂的电化学活性。通过阻抗光谱的弛豫时间分布分析,研究了二氧化碳的电解机理。在施加 2.5 V 电位和 800 °C 温度条件下,Ni-LSCT 与 GDC(Gd0.1Ce0.9O1.95)电解质可产生 3.89 A cm-2 的超高还原电流密度,具有更高的电解二氧化碳活性。在 SOEC 和 GDC 电解质中,掺杂镍是控制电化学性能和催化活性的关键因素,而高离子电导率则进一步促进了镍的掺杂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


