Factors contributing to the clinical effectiveness of imeglimin monotherapy in Japanese patients with type 2 diabetes mellitus
Abstract
Aims/Introduction
To investigate the effect of patient characteristics on imeglimin effectiveness in Japanese patients with type 2 diabetes mellitus.
Materials and Methods
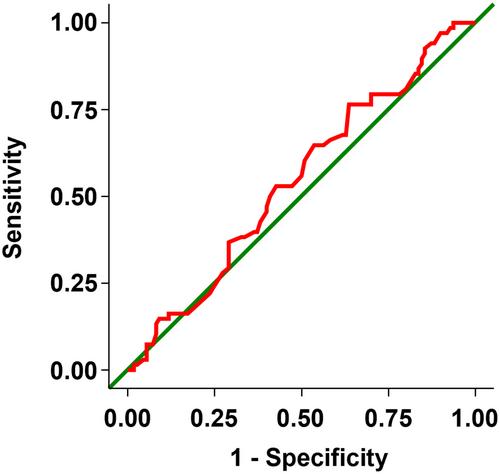
Data were pooled from two randomized, placebo-controlled, 24-week, double-blind studies of imeglimin monotherapy in Japanese adults with type 2 diabetes mellitus, with the proportion of responders (glycated hemoglobin [HbA1c] < 7.0%) and sustained responders (i.e., achieved and maintained response) in the imeglimin 1,000 mg twice daily group calculated at each visit. Patient factors significantly (P < 0.05) correlated with response were explored through multivariate logistic regression. Subgroup analyses compared the efficacy of imeglimin in patients with a HbA1c improvement less than or equal to −0.3% (early responders) versus greater than −0.3% (early non-responders) at week 4.
Results
A total of 38.0% of imeglimin-treated patients and 7.2% of placebo-treated patients were responders (P < 0.001, number needed to treat = 4). The proportion of sustained responders at weeks 4, 8, 12, 16 and 20 was 10.6, 19.0, 24.0, 25.7 and 29.1%, respectively (>70% of responders at each visit). Improvements in HbA1c and fasting glucose were significantly greater in early responders versus early non-responders from week 4; between-group differences remained significant to week 24. Older age (odds ratio 1.09, 95% confidence interval 1.04–1.14; P < 0.001); treatment-naïve status vs previous treatment (odds ratio 3.70, 95% confidence interval 1.55–8.82; P = 0.003), and lower baseline HbA1c (odds ratio 0.06, 95% confidence interval 0.02–0.16; P < 0.001) predicted response.
Conclusions
A significantly higher proportion of patients receiving imeglimin 1,000 mg twice daily monotherapy were responders versus placebo. Most (>70%) were sustained responders, suggesting that response is fairly predictable. Older age, treatment-naïve status and early treatment response significantly predicted imeglimin effectiveness.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


