General and Scalable Synthesis of 2-Aryl and 2-Alkyl Pyrimidines via an Electronically Tuned SNAr Approach
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An efficient SNAr approach for generating a wide array of 2-aryl and 2-alkyl pyrimidines in good to high yields was developed. This methodology does not require precious metal catalysts and is compatible with aryl, heteroaryl, and alkyl magnesium halides as nucleophiles. This process is scalable and performed at room temperature well below the temperature of the competing decomposition of the activated 2-tert-butyl sulfonyl pyrimidine electrophile.
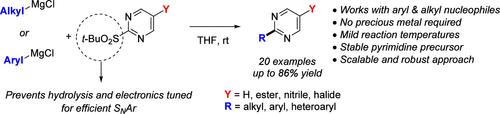
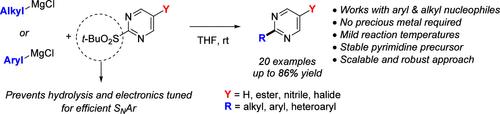
通过电子调谐 SNAr 方法通用和可扩展地合成 2-芳基和 2-烷基嘧啶。
本研究开发了一种高效的 SNAr 方法,可以高产率生成多种 2-芳基和 2-烷基嘧啶。这种方法不需要贵金属催化剂,而且与作为亲核体的芳基、杂芳基和烷基卤化镁兼容。该工艺具有可扩展性,可在室温下进行,其温度远低于活化的 2-叔丁基磺酰基嘧啶亲电子体的竞争分解温度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


