IGF2BP2-modified circular RNA circCHD7 promotes endometrial cancer progression via stabilizing PDGFRB and activating JAK/STAT signaling pathway
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Circular RNAs (circRNAs) represent a class of covalently closed, single-stranded RNAs and have been linked to cancer progression. N6-methyladenosine (m6A) methylation is a ubiquitous RNA modification in cancer cells. Increasing evidence suggests that m6A can mediate the effects of circRNAs in cancer biology. In contrast, the post-transcriptional systems of m6A and circRNA in the progression of endometrial cancer (EC) remain obscure. The current study identified a novel circRNA with m6A modification, hsa_circ_0084582 (circCHD7), which was upregulated in EC tissues. Functionally, circCHD7 was found to promote the proliferation of EC cells. Mechanistically, circCHD7 interacted with insulin-like growth factor 2 mRNA-binding protein (IGF2BP2) to amplify its enrichment. Moreover, circCHD7 increased the mRNA stability of platelet-derived growth factor receptor beta (PDGFRB) in an m6A-dependent manner, thereby enhancing its expression. In addition, the circCHD7/IGF2BP2/PDGFRB axis activated the JAK/STAT signaling pathway and promoted EC cell proliferation. In conclusion, these findings provide new insights into the regulation of circRNA-mediated m6A modification, and the new “circCHD7-PDGFRB” model of regulation offers new perspectives on circCHD7 as a potential target for EC therapy.
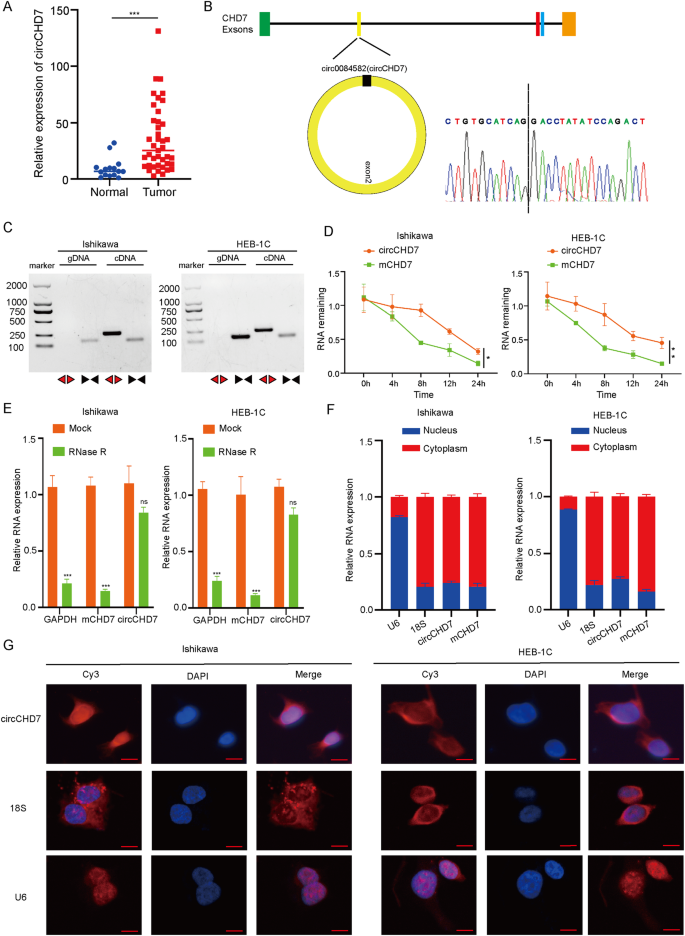
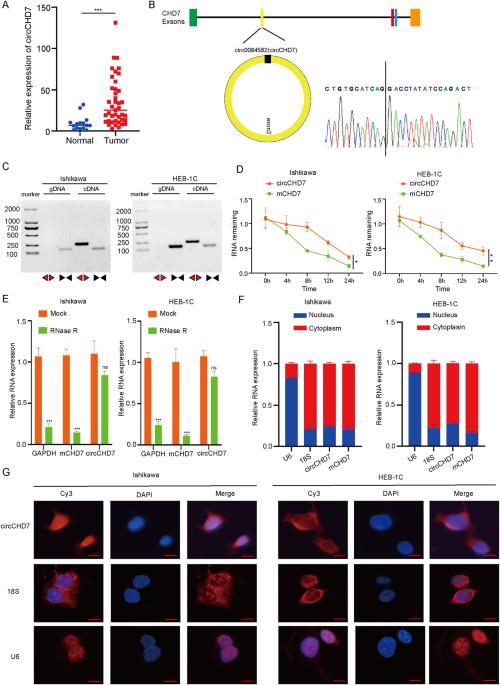
IGF2BP2修饰的环状RNA circCHD7通过稳定PDGFRB和激活JAK/STAT信号通路促进子宫内膜癌的进展。
环状 RNA(circRNA)是一类共价封闭的单链 RNA,与癌症进展有关。N6-甲基腺苷(m6A)甲基化是癌细胞中一种普遍存在的 RNA 修饰。越来越多的证据表明,m6A 可以介导 circRNA 在癌症生物学中的作用。相比之下,m6A和circRNA在子宫内膜癌(EC)进展过程中的转录后系统仍然模糊不清。目前的研究发现了一种具有m6A修饰的新型circRNA--hsa_circ_0084582(circCHD7),它在EC组织中上调。从功能上看,circCHD7 能促进心肌细胞的增殖。从机理上讲,circCHD7 与胰岛素样生长因子 2 mRNA 结合蛋白(IGF2BP2)相互作用,扩大了其富集。此外,circCHD7 以 m6A 依赖性方式增加了血小板衍生生长因子受体 beta(PDGFRB)的 mRNA 稳定性,从而提高了其表达。此外,circCHD7/IGF2BP2/PDGFRB 轴激活了 JAK/STAT 信号通路,促进了 EC 细胞增殖。总之,这些发现为研究circRNA介导的m6A修饰调控提供了新的视角,而新的 "circCHD7-PDGFRB "调控模型为将circCHD7作为EC治疗的潜在靶点提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


