Sex differences in cholinergic signaling affect functional outcomes for theta–gamma coordination in hippocampal subcircuits following experimental febrile status epilepticus
Abstract
Objective
Sex determines cognitive outcome in animal models of early life seizure, where males exhibit impaired hippocampal-dependent learning and memory compared with females. The physiological underpinnings of this sex effect are unclear. Cholinergic signaling is essential for the generation of hippocampal oscillations, and supplementation of cholinergic precursors prior to status epilepticus in immature male rats prevents subsequent memory deficits. We hypothesized that there are sex differences in acetylcholine circuits and their response to experimental febrile status epilepticus (eFSE).
Methods
eFSE was induced in male and female rat pups. We transversed the hippocampus of postnatal day >60 control (CTL) and eFSE rats with a 64-channel laminar silicon probe to assay cholinergic-dependent theta oscillations under urethane anesthesia. Local field potential properties were compared during (1) baseline sensory stimulation, (2) pharmacological stimulation via acetylcholine reuptake blockade, and (3) sensory stimulation after muscarinic acetylcholine receptor block (atropine).
Results
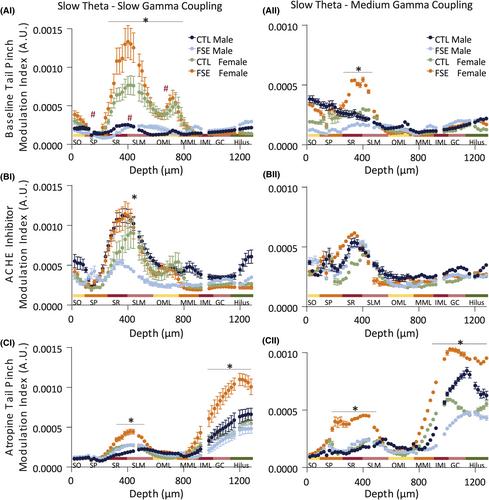
In all groups, a baseline tail pinch could elicit theta oscillations via corticohippocampal synaptic input. Following atropine, a tail pinch response could no longer be elicited in CTL male, CTL female, or eFSE female rats. In contrast, induced slow theta power in eFSE males after atropine was not decreased to spontaneous levels. Analysis of oscillation bandwidths revealed sex differences in acetylcholine modulation of theta frequency and slow gamma frequency and power. This study also identified significant effects of both sex and eFSE on baseline theta–gamma comodulation, indicating a loss of coupling in eFSE males and a potential gain of function in eFSE females.
Significance
There are differences in cholinergic modulation of theta and gamma signal coordination between male and female rats. These differences may underlie worse cognitive outcomes in males following eFSE. Promoting the efficacy of muscarinic acetylcholine signaling prior to or following early life seizures could elucidate a mechanism for the temporal discoordination of neural signals within and between hippocampus and neocortex and provide a novel therapeutic approach for improving cognitive outcomes.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


