The role of plasmacytoid dendritic cells (pDCs) in immunity during viral infections and beyond
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
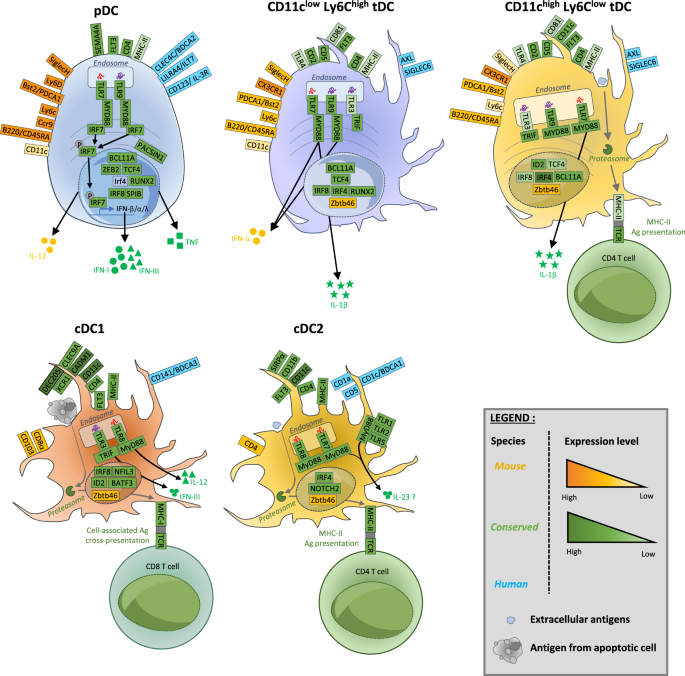
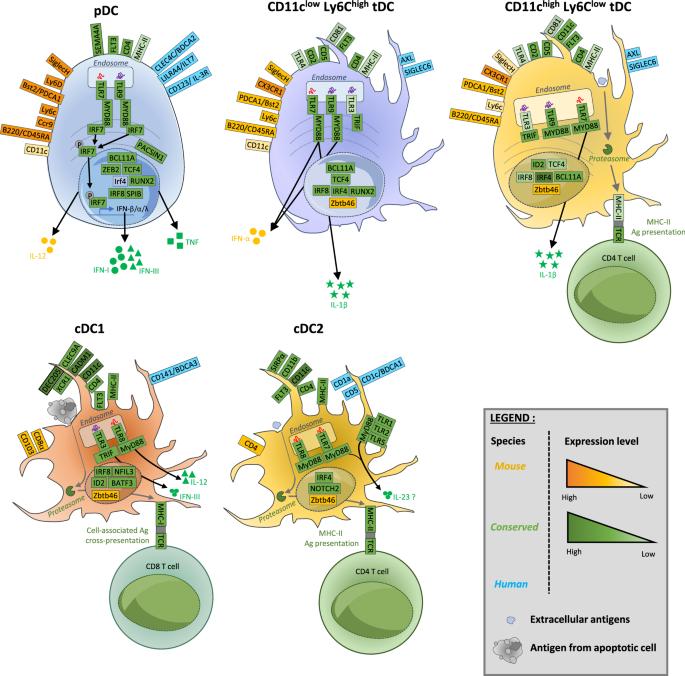
Type I and III interferons (IFNs) are essential for antiviral immunity and act through two different but complimentary pathways. First, IFNs activate intracellular antimicrobial programs by triggering the upregulation of a broad repertoire of viral restriction factors. Second, IFNs activate innate and adaptive immunity. Dysregulation of IFN production can lead to severe immune system dysfunction. It is thus crucial to identify and characterize the cellular sources of IFNs, their effects, and their regulation to promote their beneficial effects and limit their detrimental effects, which can depend on the nature of the infected or diseased tissues, as we will discuss. Plasmacytoid dendritic cells (pDCs) can produce large amounts of all IFN subtypes during viral infection. pDCs are resistant to infection by many different viruses, thus inhibiting the immune evasion mechanisms of viruses that target IFN production or their downstream responses. Therefore, pDCs are considered essential for the control of viral infections and the establishment of protective immunity. A thorough bibliographical survey showed that, in most viral infections, despite being major IFN producers, pDCs are actually dispensable for host resistance, which is achieved by multiple IFN sources depending on the tissue. Moreover, primary innate and adaptive antiviral immune responses are only transiently affected in the absence of pDCs. More surprisingly, pDCs and their IFNs can be detrimental in some viral infections or autoimmune diseases. This makes the conservation of pDCs during vertebrate evolution an enigma and thus raises outstanding questions about their role not only in viral infections but also in other diseases and under physiological conditions.
质体树突状细胞(pDCs)在病毒感染期间及以后的免疫中的作用。
I 型和 III 型干扰素(IFNs)对抗病毒免疫至关重要,它们通过两种不同但互补的途径发挥作用。首先,IFNs 通过触发病毒限制因子的上调,激活细胞内的抗微生物程序。其次,IFNs 可激活先天性和适应性免疫。IFN 生成失调可导致严重的免疫系统功能障碍。因此,确定 IFNs 的细胞来源、作用及其调控方式并描述其特征至关重要,以促进其有益作用并限制其有害作用,这可能取决于感染或患病组织的性质,我们将对此进行讨论。类质体树突状细胞(pDCs)能在病毒感染期间产生大量的所有 IFN 亚型。pDCs 能抵抗多种不同病毒的感染,从而抑制病毒针对 IFN 产生或其下游反应的免疫逃避机制。因此,pDCs 被认为是控制病毒感染和建立保护性免疫的关键。一项详尽的文献调查显示,在大多数病毒感染中,尽管 pDCs 是 IFN 的主要产生者,但它们对宿主的抵抗力实际上是可有可无的,宿主的抵抗力是由不同组织的多种 IFN 来源实现的。此外,在缺乏 pDCs 的情况下,主要的先天性和适应性抗病毒免疫反应只会受到短暂的影响。更令人惊讶的是,pDCs 及其 IFNs 在某些病毒感染或自身免疫性疾病中可能是有害的。这使得 pDCs 在脊椎动物进化过程中的保存成为一个谜,并因此提出了关于它们不仅在病毒感染中,而且在其他疾病和生理条件下的作用的悬而未决的问题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


