Cancer associated fibroblast secreted miR-432-5p targets CHAC1 to inhibit ferroptosis and promote acquired chemoresistance in prostate cancer
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
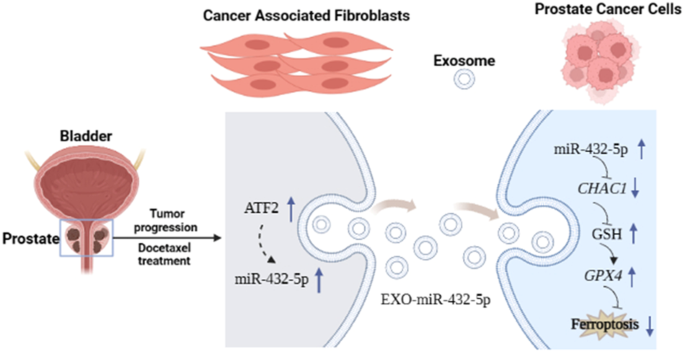
Prostate cancer (PCa) ranks as the sixth most serious male malignant disease globally. While docetaxel (DTX) chemotherapy is the standard treatment for advanced PCa patients with distant metastasis, some individuals exhibit insensitivity or resistance to DTX. Cancer-associated fibroblasts (CAFs) play a pivotal role as stromal cells within the tumor microenvironment, influencing tumor development, progression, and drug resistance through exosomes. Ferroptosis, a novel form of programmed cell death, is characterized by intracellular iron accumulation that triggers lipid peroxidation, ultimately leading to cell demise. To delve into the potential mechanisms of chemotherapy resistance in prostate cancer, our research delved into the impact of CAF-derived exosomes on ferroptosis. Our findings revealed that CAF exosomes hindered the buildup of lipid reactive oxygen species (ROS) in prostate cancer cells induced by erastin, as well as mitigated erastin-induced mitochondrial damage, thereby impeding iron-induced cell death in prostate cancer cells. Furthermore, miR-432-5p was identified to diminish glutathione (GSH) consumption by targeting CHAC1, consequently inhibiting ferroptosis in prostate cancer cells. Our study found that miR-432-5p, originating from cancer-associated fibroblast (CAF) exosomes, suppresses ferroptosis by targeting CHAC1, thereby increasing resistance to docetaxel (DTX) in PCa. This research introduces a novel approach to address resistance to DTX.
癌症相关成纤维细胞分泌的miR-432-5p靶向CHAC1,抑制前列腺癌的铁变态反应并促进获得性化疗耐药性。
前列腺癌(PCa)是全球第六大男性恶性肿瘤。虽然多西他赛(DTX)化疗是治疗有远处转移的晚期前列腺癌患者的标准疗法,但有些患者对 DTX 表现出不敏感或耐药性。癌症相关成纤维细胞(CAFs)作为肿瘤微环境中的基质细胞发挥着关键作用,通过外泌体影响肿瘤的发展、恶化和耐药性。铁凋亡是一种新型的程序性细胞死亡形式,其特点是细胞内铁积累引发脂质过氧化,最终导致细胞死亡。为了深入研究前列腺癌化疗耐药性的潜在机制,我们的研究深入探讨了CAF衍生的外泌体对铁突变的影响。我们的研究结果表明,CAF外泌体阻碍了厄拉斯汀诱导的前列腺癌细胞中脂质活性氧(ROS)的积累,并减轻了厄拉斯汀诱导的线粒体损伤,从而阻碍了铁诱导的前列腺癌细胞死亡。此外,研究还发现,miR-432-5p 可通过靶向 CHAC1 减少谷胱甘肽(GSH)的消耗,从而抑制前列腺癌细胞的铁变态反应。我们的研究发现,源自癌症相关成纤维细胞(CAF)外泌体的miR-432-5p能通过靶向CHAC1抑制铁凋亡,从而增加PCa对多西他赛(DTX)的耐药性。这项研究提出了一种解决DTX耐药性的新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


