Merging 2,3-butanedione and N-hydroxysuccinimide as visible-light-enabled hydrogen atom transfer catalysts for CC double bond cleavage of 2-cyanoaryl acrylamides toward the synthesis of 4-amino-2-quinolones†
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The CC double bond cleavage of 2-cyanoaryl acrylamides through merging 2,3-butanedione and N-hydroxysuccinimide as visible-light-enabled hydrogen atom transfer catalysts is effectively established for generating functionalized 4-amino-2-quinolones under metal-free and redox neutral conditions. Detailed mechanism studies indicate that the solvent 1,3-dioxolane offers the crucial 1,3-dioxolan-2-yl radical facilitated by the in situ formed H-atom abstracting species, and 2-alkenyl-1,3-dioxolane is probably another product in this photocatalytic protocol.
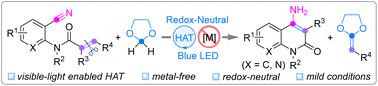
将 2,3-丁二酮和 N-羟基琥珀酰亚胺合并为可见光驱动的氢原子转移催化剂,用于从 2-氰基芳香族丙烯酰胺到 4-氨基-2-喹啉酮的 C=C 双键裂解反应
在无金属和氧化还原中性条件下,通过合并 2,3-丁二酮和 N-羟基琥珀酰亚胺作为可见光驱动的氢原子转移催化剂,有效地建立了 2-氰基芳基丙烯酰胺的 C=C 双键裂解,从而生成功能化的 4-氨基-2-喹啉酮。详细的机理研究表明,溶剂 1,3-二氧戊环在原位形成的氢原子抽取物种的促进下提供了关键的 1,3-二氧戊环-2-基自由基,而 2-烯基-1,3-二氧戊环可能是该光催化方案中的另一种产物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


