Theoretical insights into the chiral separation of levobunolol
IF 1.5
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
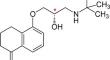
In this study, we conducted a theoretical investigation to elucidate the chiral recognition mechanisms of polysaccharide-derived stationary phase for the bunolol β-blocker. DFT calculations provided structural and energetic insights, successfully explaining chiral discrimination and enantiomeric elution order obtained in previous HPLC experiments. Our analysis highlighted the crucial role of hydrogen bonding and π–π stacking interactions in determining the relative stability of the diastereomeric complexes formed between the bunolol and chiral selector.
左旋布诺洛尔手性分离的理论见解
在本研究中,我们进行了一项理论研究,以阐明布诺洛尔β受体阻滞剂多糖衍生固定相的手性识别机制。DFT 计算提供了结构和能量方面的见解,成功解释了之前 HPLC 实验中获得的手性识别和对映体洗脱顺序。我们的分析强调了氢键和 π-π 堆积相互作用在决定布诺洛尔和手性选择剂之间形成的非对映异构体复合物的相对稳定性方面的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Theoretical Chemistry Accounts
化学-物理化学
CiteScore
3.40
自引率
0.00%
发文量
74
审稿时长
3.8 months
期刊介绍:
TCA publishes papers in all fields of theoretical chemistry, computational chemistry, and modeling. Fundamental studies as well as applications are included in the scope. In many cases, theorists and computational chemists have special concerns which reach either across the vertical borders of the special disciplines in chemistry or else across the horizontal borders of structure, spectra, synthesis, and dynamics. TCA is especially interested in papers that impact upon multiple chemical disciplines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


