A synthesis of dibenzo[b,h][1,5]naphthyridin-7(12H)-ones: the Pictet–Spengler reaction vs a rearrangement of 4-phenyl[1,3]oxazolo[4,5-c]quinolines
引用次数: 0
Abstract
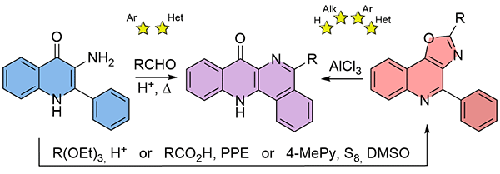
Two approaches were proposed to the synthesis of dibenzo[b,h][1,5]naphthyridin-7(12H)-ones. The one-step method based on the Pictet–Spengler reaction of 3-amino-2-phenylquinolin-4(1H)-one with aromatic aldehydes requires heating in a strong acidic media, which leads to significant limitations on the possible products. The second approach is based on the conversion of 3-amino-2-phenylquinolin-4(1H)-one to 4-phenyl[1,3]oxazolo[4,5-c]quinolines followed by rearrangement under the action of AlCl3. A significant advantage of the two-step synthesis is the possibility of obtaining a wider range of dibenzo[b,h][1,5]naphthyridin-7(12H)-ones, including those not available using the Pictet–Spengler reaction.
二苯并[b,h][1,5]萘啶-7(12H)-酮的合成:皮克泰-斯彭勒反应与 4-苯基[1,3]恶唑并[4,5-c]喹啉的重排反应
提出了合成二苯并[b,h][1,5]萘啶-7(12H)-酮的两种方法。基于 3-氨基-2-苯基喹啉-4(1H)-酮与芳香醛的 Pictet-Spengler 反应的一步法需要在强酸性介质中加热,这导致可能的产物受到很大限制。第二种方法是将 3-氨基-2-苯基喹啉-4(1H)-酮转化为 4-苯基[1,3]恶唑并[4,5-c]喹啉,然后在 AlCl3 的作用下进行重排。两步合成法的一个显著优点是可以获得范围更广的二苯并[b,h][1,5]萘啶-7(12H)-酮,包括那些无法通过皮克泰-斯彭勒反应获得的二苯并[b,h][1,5]萘啶-7(12H)-酮。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


