Remote-Carbonyl-Directed Consecutive Arylation of Terminal Alkenes for the Synthesis of Tetrasubstituted Olefins
引用次数: 0
Abstract
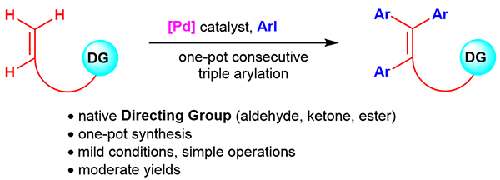
The highly efficient synthesis of all-carbon tetrasubstituted olefins has been concerned for decades, especially for multi-aryl substituted olefins which widely used in functional organic materials and pharmaceuticals. This work presents a carbonyl-directed palladium-catalyzed consecutive arylation of terminal alkenes with aryl iodides under mild conditions, a series of triarylated tetrasubstituted olefins were obtained in moderate yields. Because a weak chelation effect is generally difficult to support such a thorough tri-fold Heck arylation, and the β-trans-selective alkenyl C–H activation cannot be achieved via a twisted endo-metallacyclic intermediate, thus, the key to success is the compatibility between several mechanisms, including Heck, C–H activation and E,Z-isomerization. Here, the judicious selection of a flexible-alkyl-chain-tethered carbonyl group seems to be critical, it provides a proper chelation effect that not only assists a distal alkenyl functionalization or isomerization, but also avoids byproducts caused by other possible β-H elimination or migration. The strategy developed herein greatly streamlines the preparation of the target molecules, and the protocol covers a range of readily available terminal alkenes bearing native directing groups (e.g., aldehyde, ketone and ester), and aryl iodides.
末端烯烃的远程羰基定向连续芳基化以合成四代烯烃
几十年来,全碳四取代烯烃的高效合成一直备受关注,尤其是多芳基取代烯烃,广泛应用于功能有机材料和医药领域。本研究提出了一种在温和条件下由羰基引导的钯催化末端烯烃与芳基碘化物的连续芳基化反应,以中等产率获得了一系列三芳基化的四取代烯烃。由于弱螯合作用一般难以支持如此彻底的三折 Heck 芳化反应,而且无法通过扭曲的内金属环中间体实现 β-反式选择性烯基 C-H 活化,因此,成功的关键在于 Heck、C-H 活化和 E,Z 异构化等几种机制之间的兼容性。在这里,明智地选择柔性烷基链系羰基似乎至关重要,它能提供适当的螯合效果,不仅有助于远端烯基官能化或异构化,还能避免其他可能的 β-H 消除或迁移所造成的副产物。本文开发的策略大大简化了目标分子的制备过程,而且该方案涵盖了一系列容易获得的带有原生定向基团(如醛、酮和酯)的末端烯烃和芳基碘化物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


