Ex-Chiral-pool Synthesis of Optically Active 4-Alkylidene-Tetrahydroisoquinolines – Key Intermediates for Crinane Alkaloid Total Syntheses
引用次数: 0
Abstract
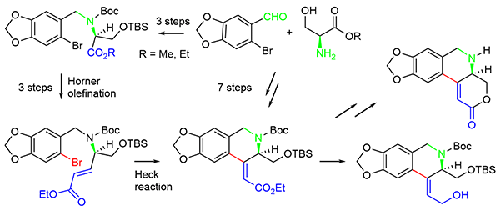
A seven-step ex-chiral-pool synthesis of optically active 4-alkylidene-tetrahydroisoquinolines was developed. Starting from 6-bromopiperonal and (S)-serine esters N-benzylation via reductive amination gave enantiopure N-piperonyl serine esters. Subsequent NH and OH protection delivered defined (S)-serine building blocks. Best results to achieve the conversion into the corresponding serinal were obtained via a two-step sequence of NaBH4/LiCl reduction and subsequent TEMPO oxidation. Then, chain elongation using the Masamune-Roush variant of the Horner olefination afforded (E)-ethyl 4-(N-6-bromopiperonyl) substituted pentenoates with high yields. Intramolecular Heck cyclization employing the Herrmann-Beller catalyst enabled generation of enantiopure 4-(2-ethoxycarbonylmethylidene)-tetrahydroisoquinoline building blocks with high (Z) selectivity. Subsequent selected functional group transformations gave carbinols and lactones, which will be used as key intermediates in crinane alkaloid total syntheses.
具有光学活性的 4-亚烷基四氢异喹啉的手性池外合成--铬烷类生物碱全合成的关键中间体
本研究开发了一种具有光学活性的 4-亚烷基四氢异喹啉的七步手性池外合成法。从 6-溴哌丙醛和 (S)- 丝氨酸酯开始,通过还原胺化进行 N-苄基化,得到对映体纯的 N-哌丙基丝氨酸酯。随后的 NH 和 OH 保护提供了确定的 (S)- 丝氨酸结构单元。通过 NaBH4/LiCl 还原和随后的 TEMPO 氧化两步顺序,获得了转化为相应丝氨酸的最佳结果。然后,利用 Horner 烯化反应的 Masamune-Roush 变体进行链延伸,高产率地获得了 (E)-ethyl 4-(N-6-溴哌酰基) 取代的戊烯酸酯。利用 Herrmann-Beller 催化剂进行分子内 Heck 环化反应,可以生成对映体纯的 4-(2-乙氧羰基亚甲基)-四氢异喹啉构筑模块,并具有高 (Z) 选择性。随后的选择官能团转化产生了焦醇和内酯,它们将作为关键的中间体用于克林烷生物碱的全合成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


