RAF1 facilitates KIT signaling and serves as a potential treatment target for gastrointestinal stromal tumor
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
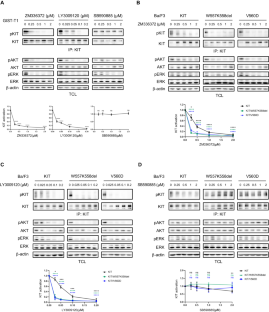
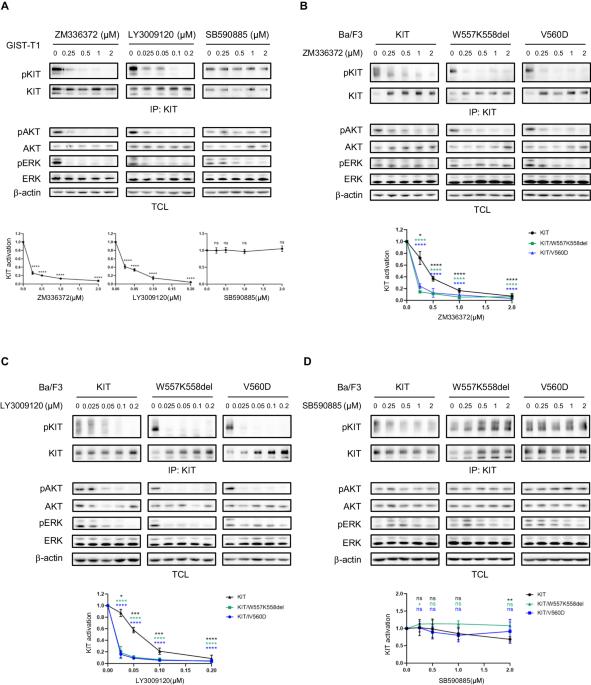
The aberrant activation of RAS/RAF/MEK/ERK signaling is important for KIT mutation-mediated tumorigenesis of gastrointestinal stromal tumor (GIST). In this study, we found that inhibition of RAF1 suppresses the activation of both wild-type KIT and primary KIT mutations in GIST, with primary KIT mutations showing greater sensitivity. This suggests a positive feedback loop between KIT and RAF1, wherein RAF1 facilitates KIT signaling. We further demonstrated that RAF1 associates with KIT and the kinase activity of RAF1 is necessary for its contribution to KIT activation. Accordingly, inhibition of RAF1 suppressed cell survival, proliferation, and cell cycle progression in vitro mediated by both wild-type KIT and primary KIT mutations. Inhibition of RAF1 in vivo suppressed GIST growth in a transgenic mouse model carrying germline KIT/V558A mutation, showing a similar treatment efficiency as imatinib, the first-line targeted therapeutic drug of GIST, while the combination use of imatinib and RAF1 inhibitor further suppressed tumor growth. Acquisition of drug-resistant secondary mutation of KIT is a major cause of treatment failure of GIST following targeted therapy. Like wild-type KIT and primary KIT mutations, inhibition of RAF1 suppressed the activation of secondary KIT mutation, and the cell survival, proliferation, cell cycle progression in vitro, and tumor growth in vivo mediated by secondary KIT mutation. However, the activation of secondary KIT mutation is less dependent on RAF1 compared with that of primary KIT mutations. Taken together, our results revealed that RAF1 facilitates KIT signaling and KIT mutation-mediated tumorigenesis of GIST, providing a rationale for further investigation into the use of RAF1 inhibitors alone or in combination with KIT inhibitor in the treatment of GIST, particularly in cases resistant to KIT inhibitors.
RAF1 促进 KIT 信号转导,是胃肠道间质瘤的潜在治疗靶点。
RAS/RAF/MEK/ERK信号的异常激活是KIT突变介导的胃肠道间质瘤(GIST)肿瘤发生的重要原因。在这项研究中,我们发现抑制 RAF1 可抑制 GIST 中野生型 KIT 和原发性 KIT 突变的激活,而原发性 KIT 突变表现出更高的敏感性。这表明 KIT 和 RAF1 之间存在正反馈循环,其中 RAF1 促进了 KIT 信号转导。我们进一步证实,RAF1 与 KIT 关联,RAF1 的激酶活性是其促进 KIT 激活的必要条件。因此,抑制 RAF1 可抑制野生型 KIT 和原发性 KIT 突变介导的体外细胞存活、增殖和细胞周期进展。在体内抑制 RAF1 可抑制携带 KIT/V558A 基因突变的转基因小鼠模型中 GIST 的生长,治疗效果与 GIST 的一线靶向治疗药物伊马替尼相似,而伊马替尼和 RAF1 抑制剂的联合使用可进一步抑制肿瘤生长。KIT二次突变耐药是GIST靶向治疗失败的主要原因。与野生型KIT和原发性KIT突变一样,RAF1抑制剂抑制了继发性KIT突变的激活,以及由继发性KIT突变介导的体外细胞存活、增殖、细胞周期进展和体内肿瘤生长。然而,与原发性 KIT 突变相比,继发性 KIT 突变的活化对 RAF1 的依赖性较低。综上所述,我们的研究结果表明,RAF1 促进了 KIT 信号转导和 KIT 突变介导的 GIST 肿瘤发生,为进一步研究单独使用 RAF1 抑制剂或与 KIT 抑制剂联合使用来治疗 GIST(尤其是对 KIT 抑制剂耐药的病例)提供了依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


