Cost-effectiveness analysis of CYP3A5 genotype-guided tacrolimus dosing in solid organ transplantation using real-world data
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 0
Abstract
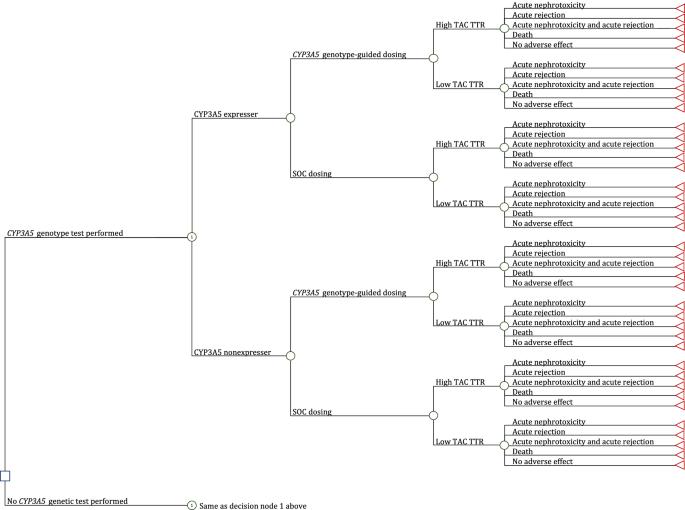
The objective of this study was to estimate the cost-effectiveness of CYP3A5 genotype-guided tacrolimus dosing in kidney, liver, heart, and lung transplant recipients relative to standard of care (SOC) tacrolimus dosing, from a US healthcare payer perspective. We developed decision-tree models to compare economic and clinical outcomes between CYP3A5 genotype-guided and SOC tacrolimus therapy in the first six months post-transplant. We derived inputs for CYP3A5 phenotype frequencies and physician use of genotype test results to inform clinical care from literature; tacrolimus exposure [high vs low tacrolimus time in therapeutic range using the Rosendaal algorithm (TAC TTR-Rosendaal)] and outcomes (incidences of acute tacrolimus nephrotoxicity, acute cellular rejection, and death) from real-world data; and costs from the Medicare Fee Schedule and literature. We calculated cost per avoided event and performed sensitivity analyses to evaluate the robustness of the results to changes in inputs. Incremental costs per avoided event for CYP3A5 genotype-guided vs SOC tacrolimus dosing were $176,667 for kidney recipients, $364,000 for liver recipients, $12,982 for heart recipients, and $93,333 for lung recipients. The likelihood of CYP3A5 genotype-guided tacrolimus dosing leading to cost-savings was 19.8% in kidney, 32.3% in liver, 51.8% in heart, and 54.1% in lung transplant recipients. Physician use of genotype results to guide clinical care and the proportion of patients with a high TAC TTR-Rosendaal were key parameters driving the cost-effectiveness of CYP3A5 genotype-guided tacrolimus therapy. Relative to SOC, CYP3A5 genotype-guided tacrolimus dosing resulted in a slightly greater benefit at a higher cost. Further economic evaluations examining intermediary outcomes (e.g., dose modifications) are needed, particularly in populations with higher frequencies of CYP3A5 expressers.
利用真实世界的数据对CYP3A5基因型指导下他克莫司在实体器官移植中的剂量进行成本效益分析。
本研究旨在从美国医疗支付方的角度估算肾、肝、心和肺移植受者在 CYP3A5 基因型指导下服用他克莫司与标准护理(SOC)服用他克莫司的成本效益。我们开发了决策树模型,以比较 CYP3A5 基因型指导下的他克莫司治疗与标准护理下的他克莫司治疗在移植后头六个月的经济和临床结果。我们从文献中获得了 CYP3A5 表型频率和医生使用基因型检测结果指导临床治疗的输入数据;从真实世界数据中获得了他克莫司暴露量[使用罗森达尔算法(TAC TTR-Rosendaal)计算的治疗范围内他克莫司时间高与低]和结果(急性他克莫司肾毒性、急性细胞排斥反应和死亡发生率);从医疗保险收费表和文献中获得了成本。我们计算了每次避免事件的成本,并进行了敏感性分析,以评估结果对输入变化的稳健性。CYP3A5 基因型指导与 SOC 他克莫司给药相比,肾脏受者每次避免事件的增量成本为 176667 美元,肝脏受者为 364000 美元,心脏受者为 12982 美元,肺部受者为 93333 美元。肾移植受者在 CYP3A5 基因型指导下服用他克莫司而节省费用的可能性为 19.8%,肝移植受者为 32.3%,心脏移植受者为 51.8%,肺移植受者为 54.1%。医生使用基因型结果指导临床治疗以及TAC TTR-Rosendaal高的患者比例是影响CYP3A5基因型指导他克莫司治疗成本效益的关键参数。与 SOC 相比,CYP3A5 基因型指导下的他克莫司剂量带来的益处稍大,但成本较高。还需要对中间结果(如剂量调整)进行进一步的经济评估,尤其是在 CYP3A5 表达率较高的人群中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


