Synthesis of various 1-alkylbenzimidazole derivatives directly from 2-alkylaminonitroarenes via a two-step, one-pot procedure
IF 2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
N-Alkyl-2-nitroanilines deoxygenated with tributylphosphine form intermediate 2-(alkylamino)aryliminophosphoranes, which were subjected, without isolation, to various cyclocondensation reactions with CS2, CO2, alkyl isocyanates, acyl chlorides, anhydrides, or esters. A simple, convenient, one-pot procedure provided derivatives of unsymmetrically substituted 1-alkylbenzimidazoles functionalized at C2 in good to excellent yields. The method does not require the use of metals, sensitive catalysts, or pressure.
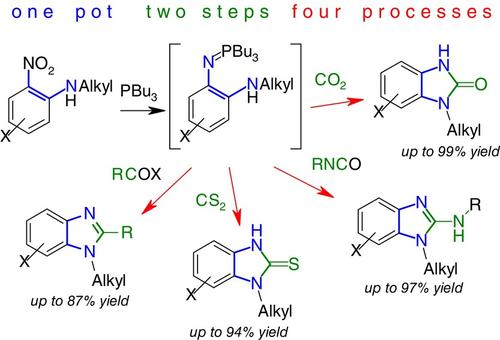
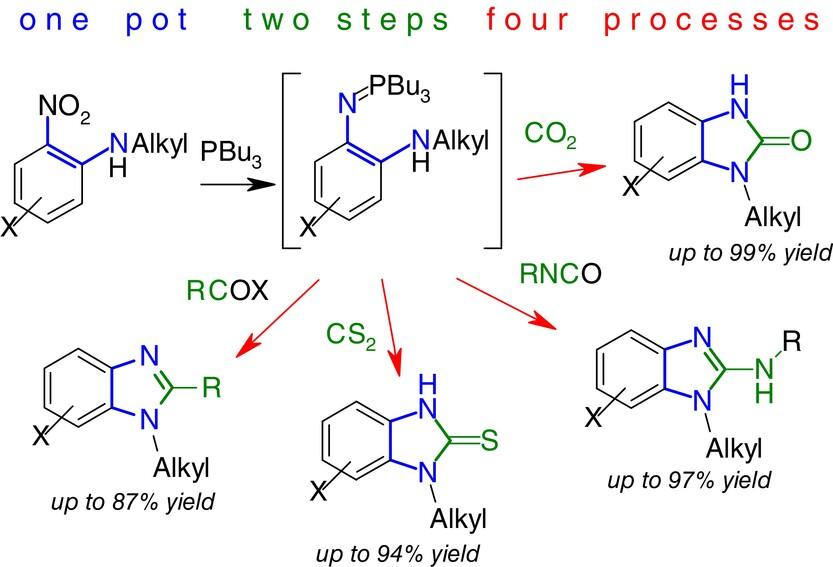
通过两步一步法直接从 2-烷基氨基硝基苯烯合成各种 1-烷基苯并咪唑衍生物
用三丁基膦脱氧的 N-烷基-2-硝基苯胺形成 2-(烷基氨基)芳基亚胺基膦中间体,这些中间体与 CS2、CO2、异氰酸烷基酯、酰基氯、酸酐或酯进行了各种环缩合反应,而没有进行分离。通过简单、方便的一锅式反应过程,可以获得在 C2 处官能化的不对称取代的 1-烷基苯并咪唑衍生物,收率良好甚至极佳。该方法无需使用金属、敏感催化剂或压力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.20
自引率
4.20%
发文量
177
审稿时长
3.9 months
期刊介绍:
The Journal of Heterocyclic Chemistry is interested in publishing research on all aspects of heterocyclic chemistry, especially development and application of efficient synthetic methodologies and strategies for the synthesis of various heterocyclic compounds. In addition, Journal of Heterocyclic Chemistry promotes research in other areas that contribute to heterocyclic synthesis/application, such as synthesis design, reaction techniques, flow chemistry and continuous processing, multiphase catalysis, green chemistry, catalyst immobilization and recycling.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


