Sevoflurane blocks KLF5-mediated transcriptional activation of ITGB2 to inhibit macrophage infiltration in hepatic ischemia/reperfusion injury
Abstract
Background
Sevoflurane (Sevo) preconditioning and postconditioning play a protective role against injury induced by hepatic ischemia/reperfusion (I/R). At the same time, the involvement of macrophage infiltration in this process and the precise mechanisms are unclear. Here, we designed this research to elucidate the protective effects of Sevo against hepatic I/R injury and the molecules involved.
Methods
The alleviating effect of Sevo on the liver injury was analyzed by liver function analysis, hematoxylin and eosin staining, Masson trichrome staining, terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate nick end labeling, western blot analysis and an enzyme-linked immunosorbent assay. An in vitro cell model was developed using alpha mouse liver 12 (AML12) cells, and the cell model was treated with oxygen–glucose deprivation and reoxygenation and Sevo. Multiple bioinformatics databases were used to screen transcriptional regulators related to hepatic I/R injury and the targets of Krueppel-like factor 5 (KLF5). KLF5 expression was artificially upregulated alone or with integrin beta-2 (ITGB2) knockdown to substantiate their involvement in Sevo-mediated hepatoprotection.
Results
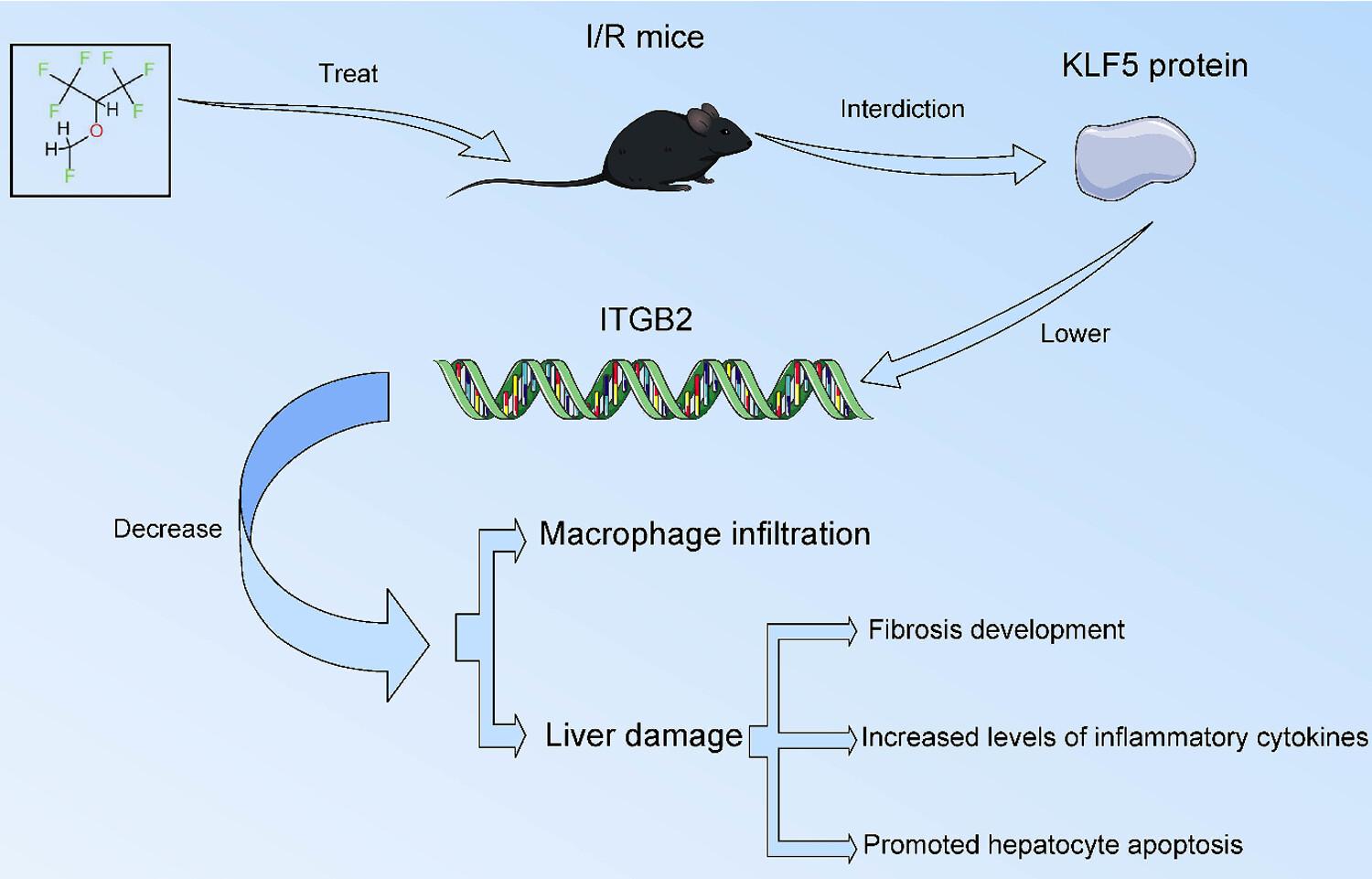
Sevo protected the liver against I/R injury by reducing cell apoptosis and inflammatory response. KLF5 was upregulated in liver tissues following I/R injury, whereas KLF5 overexpression aggravated macrophage infiltration and liver injury induced by I/R injury. KLF5 bound to the promoter of ITGB2 to enhance ITGB2 transcription. Knockdown of ITGB2 reversed the aggravation of injury caused by KLF5 overexpression in mice and AML12 cells.
Conclusions
Sevo blocked KLF5-mediated transcriptional activation of ITGB2, thereby inhibiting macrophage infiltration in hepatic I/R injury.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


