Dickkopf-1 (DKK1) drives growth and metastases in castration-resistant prostate cancer
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Metastatic castration-resistant prostate cancer (mCRPC) is associated with a poor prognosis and remains an incurable fatal disease. Therefore, the identification of molecular markers involved in cancer progression is urgently needed to develop more-effective therapies. The present study investigated the role of the Wnt signaling modulator Dickkopf-1 (DKK1) in the growth and metastatic progression of mCRPC. DKK1 silencing through siRNA and deletion via CRISPR/Cas9 editing were performed in two different metastatic castration-resistant prostate cancer cell lines (PC3 and DU145). A xenograft tumor model was used to assess tumor growth and metastases. In in vitro experiments, both DKK1 silencing and deletion reduced cell growth and migration of both cell lines. DKK1 knockout clones (DKK1-KO) exhibited cell cycle arrest, tubulin reorganization, and modulation of tumor metastasis-associated genes. Furthermore, in DKK1-KO cells, E-cadherin re-expression and its membrane co-localization with β-catenin were observed, contributing to reduced migration; Cadherin-11, known to increase during epithelial-mesenchymal transition, was down-regulated in DKK1-KO cells. In the xenograft mouse model, DKK1 deletion not only reduced tumor growth but also inhibited the formation of lung metastases. In conclusion, our findings support the key role of DKK1 in the growth and metastatic dissemination of mCRPC, both in vitro and in vivo.
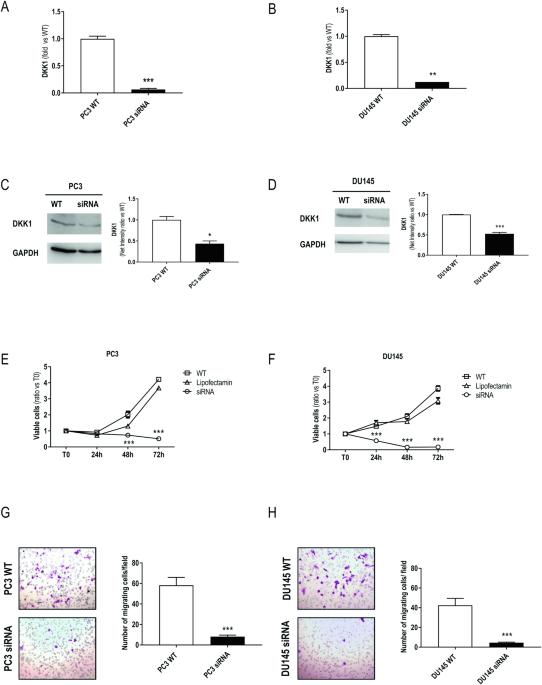
Dickkopf-1(DKK1)能促进耐受性前列腺癌的生长和转移。
转移性抗性前列腺癌(mCRPC)预后不良,仍是一种无法治愈的致命疾病。因此,迫切需要确定癌症进展的分子标记物,以开发更有效的疗法。本研究探讨了Wnt信号调节因子Dickkopf-1(DKK1)在mCRPC的生长和转移过程中的作用。研究人员在两种不同的转移性阉割耐药前列腺癌细胞系(PC3和DU145)中通过siRNA沉默DKK1,并通过CRISPR/Cas9编辑删除DKK1。异种移植肿瘤模型用于评估肿瘤生长和转移情况。在体外实验中,DKK1 的沉默和缺失都降低了两种细胞系的细胞生长和迁移。DKK1基因敲除克隆(DKK1-KO)表现出细胞周期停滞、微管蛋白重组和肿瘤转移相关基因的调控。此外,在DKK1-KO细胞中,观察到E-cadherin重新表达及其与β-catenin的膜共定位,从而导致迁移减少;已知在上皮-间质转化过程中会增加的Cadherin-11在DKK1-KO细胞中下调。在异种移植小鼠模型中,DKK1 基因缺失不仅降低了肿瘤的生长,还抑制了肺转移的形成。总之,我们的研究结果支持DKK1在mCRPC的体外和体内生长和转移扩散中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


