Julia Y Chu, Barry McCormick, Kruthika Sundaram, Gareth Hardisty, Utsa Karmakar, Caroline Pumpe, Elizabeth Krull, Christopher D Lucas, Joana Amado-Azevedo, Peter L Hordijk, Andrea Caporali, Harry Mellor, J Kenneth Baillie, Adriano G Rossi, Sonja Vermeren
下载PDF
{"title":"ARAP3 protects from excessive formylated peptide-induced microvascular leakage by acting on endothelial cells and neutrophils","authors":"Julia Y Chu, Barry McCormick, Kruthika Sundaram, Gareth Hardisty, Utsa Karmakar, Caroline Pumpe, Elizabeth Krull, Christopher D Lucas, Joana Amado-Azevedo, Peter L Hordijk, Andrea Caporali, Harry Mellor, J Kenneth Baillie, Adriano G Rossi, Sonja Vermeren","doi":"10.1002/path.6288","DOIUrl":null,"url":null,"abstract":"<p>Vascular permeability is temporarily heightened during inflammation, but excessive inflammation-associated microvascular leakage can be detrimental, as evidenced in the inflamed lung. Formylated peptides regulate vascular leakage indirectly via formylated peptide receptor-1 (FPR1)-mediated recruitment and activation of neutrophils. Here we identify how the GTPase-activating protein ARAP3 protects against formylated peptide-induced microvascular permeability via endothelial cells and neutrophils. <i>In vitro</i>, <i>Arap3</i><sup>−/−</sup> endothelial monolayers were characterised by enhanced formylated peptide-induced permeability due to upregulated endothelial FPR1 and enhanced vascular endothelial cadherin internalisation. <i>In vivo</i>, enhanced inflammation-associated microvascular leakage was observed in <i>Arap3</i><sup>−/−</sup> mice. Leakage of plasma protein into the lungs of <i>Arap3</i><sup>−/−</sup> mice increased within hours of formylated peptide administration. Adoptive transfer experiments indicated this was dependent upon ARAP3 deficiency in both immune and non-immune cells. Bronchoalveolar lavages of formylated peptide-challenged <i>Arap3</i><sup>−/−</sup> mice contained neutrophil extracellular traps (NETs). Pharmacological inhibition of NET formation abrogated excessive microvascular leakage, indicating a critical function of NETs in this context. The observation that <i>Arap3</i><sup>−/−</sup> mice developed more severe influenza suggests these findings are pertinent to pathological situations characterised by abundant formylated peptides. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 3","pages":"347-359"},"PeriodicalIF":5.6000,"publicationDate":"2024-05-11","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6288","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6288","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
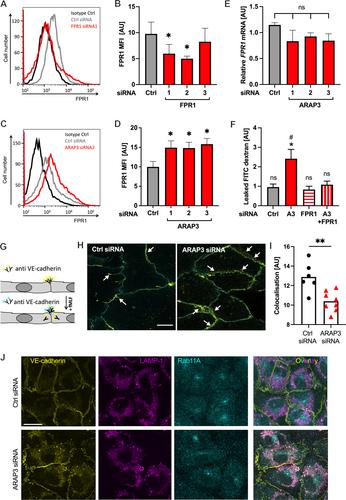
Vascular permeability is temporarily heightened during inflammation, but excessive inflammation-associated microvascular leakage can be detrimental, as evidenced in the inflamed lung. Formylated peptides regulate vascular leakage indirectly via formylated peptide receptor-1 (FPR1)-mediated recruitment and activation of neutrophils. Here we identify how the GTPase-activating protein ARAP3 protects against formylated peptide-induced microvascular permeability via endothelial cells and neutrophils. In vitro , Arap3 −/− endothelial monolayers were characterised by enhanced formylated peptide-induced permeability due to upregulated endothelial FPR1 and enhanced vascular endothelial cadherin internalisation. In vivo , enhanced inflammation-associated microvascular leakage was observed in Arap3 −/− mice. Leakage of plasma protein into the lungs of Arap3 −/− mice increased within hours of formylated peptide administration. Adoptive transfer experiments indicated this was dependent upon ARAP3 deficiency in both immune and non-immune cells. Bronchoalveolar lavages of formylated peptide-challenged Arap3 −/− mice contained neutrophil extracellular traps (NETs). Pharmacological inhibition of NET formation abrogated excessive microvascular leakage, indicating a critical function of NETs in this context. The observation that Arap3 −/− mice developed more severe influenza suggests these findings are pertinent to pathological situations characterised by abundant formylated peptides. © 2024 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
ARAP3 通过作用于内皮细胞和中性粒细胞,防止过度甲酰肽诱导的微血管渗漏。
在炎症期间,血管通透性会暂时升高,但与炎症相关的微血管渗漏过多会造成危害,这一点在发炎的肺部得到了证明。甲酰肽通过甲酰肽受体-1(FPR1)介导的中性粒细胞招募和活化间接调节血管渗漏。在这里,我们确定了 GTP 酶激活蛋白 ARAP3 如何通过内皮细胞和中性粒细胞保护甲酰肽诱导的微血管通透性。在体外,ARAP3-/-内皮单层由于内皮 FPR1 上调和血管内皮粘连蛋白内化增强,甲酰肽诱导的通透性增强。在体内,观察到 Arap3-/- 小鼠炎症相关的微血管渗漏增强。给 Arap3-/- 小鼠注射甲酰肽后数小时内,血浆蛋白渗漏到小鼠肺部的情况增加。采用转移实验表明,这取决于免疫细胞和非免疫细胞中 ARAP3 的缺乏。甲酰肽挑战 Arap3-/- 小鼠的支气管肺泡灌洗液中含有中性粒细胞胞外陷阱(NET)。药物抑制 NET 的形成可减少微血管的过度渗漏,这表明 NET 在这种情况下具有关键功能。Arap3-/-小鼠患上更严重流感的观察结果表明,这些发现与以大量甲酰肽为特征的病理情况有关。© 2024 作者。病理学杂志》由 John Wiley & Sons Ltd 代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


